On November 25, 2025, the U.S. Food and Drug Administration (FDA) approved durvalumab (Imfinzi) in combination with FLOT chemotherapy as neoadjuvant and adjuvant treatment, followed by durvalumab monotherapy, for adults with resectable gastric or gastroesophageal junction adenocarcinoma (GC/GEJC).
This approval is grounded in the phase 3 MATTERHORN trial, which investigated whether integrating immunotherapy into standard perioperative FLOT could further improve outcomes in stage II–IVA disease.
Trial Design and Methods
MATTERHORN (NCT04592913) is a large, global, randomized, double-blind, placebo-controlled phase 3 study evaluating whether adding durvalumab to standard FLOT chemotherapy improves outcomes for patients with resectable, previously untreated gastric or gastroesophageal junction adenocarcinoma. The trial was led by Dr. Yelena Y. Janjigian (Memorial Sloan Kettering Cancer Center) together with an international group of investigators across Europe, Asia, and North America.
A total of 948 patients with stage II–IVA disease were enrolled and randomized 1:1 to receive:
- Durvalumab + FLOT (neoadjuvant + adjuvant), followed by durvalumab alone
vs. - Placebo + FLOT
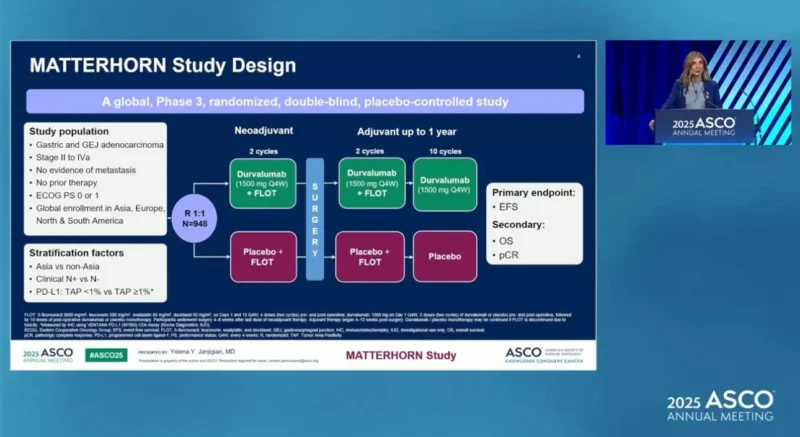
Treatment was delivered perioperatively: four cycles before surgery and four cycles after surgery, followed by durvalumab maintenance.
The primary endpoint was event-free survival (EFS) by blinded independent review.
Key secondary endpoints included overall survival (OS) and pathological complete response (pCR) assessed centrally.
The study was not designed to isolate the impact of durvalumab separately during the neoadjuvant or adjuvant phases.
Interim MATTERHORN Results
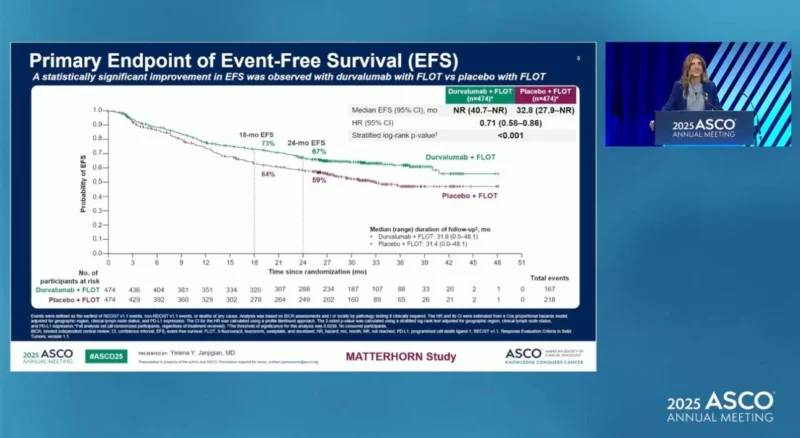
The interim efficacy analysis of MATTERHORN, presented by Dr. Yelena Y. Janjigian during the ASCO 2025 Plenary Session and published in The New England Journal of Medicine, provided the first mature readout from the 948 randomized participants—474 assigned to durvalumab and 474 to placebo—with a median follow-up of 31.5 months (interquartile range, 26.7–36.6). Durvalumab + FLOT significantly improved event-free survival, with a hazard ratio of 0.71 (95% CI, 0.58–0.86; P<0.001). Two-year EFS was 67.4% vs 58.5% with placebo.
Two-year overall survival was 75.7% with durvalumab vs 70.4% with placebo. A piecewise OS analysis showed a hazard ratio of 0.99 in months 0–12 and 0.67 after 12 months (P=0.03; boundary P<0.0001). Durvalumab improved pathological response, with pCR rates of 19.2% vs 7.2% (RR 2.69; 95% CI, 1.86–3.90).
Safety was comparable: grade 3–4 AEs occurred in 71.6% vs 71.2%, delayed surgery in 10.1% vs 10.8%, and delayed adjuvant therapy in 2.3% vs 4.6%.
In the ASCO discussion, Dr. Sam Klempner called this regimen the “new global standard” and informally referred to perioperative durvalumab + FLOT as “D-FLOT.”
Final Results
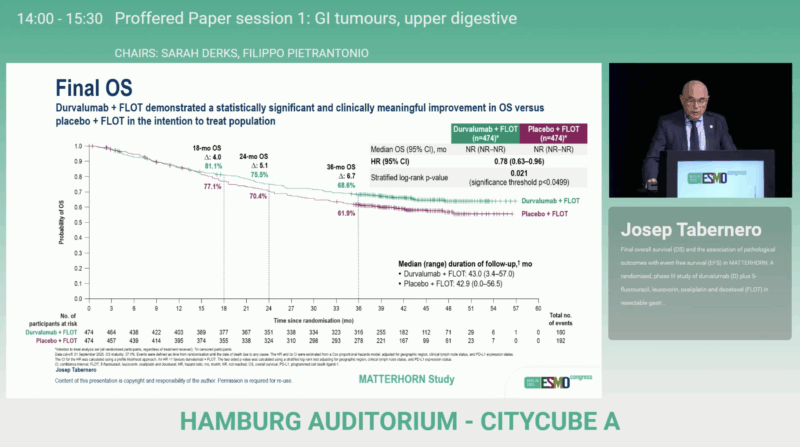
The final results of the MATTERHORN trial were presented by Professor Josep Tabernero at the ESMO Congress 2025 in Berlin, delivering the definitive overall survival data that formed the foundation of the FDA approval. The ESMO dataset fully aligned with the efficacy measures reported by the FDA, confirming consistent and clinically meaningful benefit across endpoints.
Prof. Tabernero reported that durvalumab + FLOT produced a statistically significant overall survival improvement compared with placebo + FLOT. The OS hazard ratio was 0.78 (95% CI 0.63–0.96), with median OS not reached in either group. Importantly, the survival advantage was independent of PD-L1 expression, with comparable hazard ratios across TAP <1% and TAP ≥1% subgroups.
The Berlin presentation also highlighted deeper pathological response. Patients treated with durvalumab achieved higher nodal negativity, with ypN0 rates of 58.2% versus 44.8% in the placebo arm. Tumor regression was more pronounced across all response categories, and the improvement in event-free survival remained evident regardless of whether patients achieved partial, major, or complete pathological response.
A limited set of bullets summarizing the ESMO dataset:
- Final OS benefit: HR 0.78, median OS not reached
- Consistent OS benefit in both PD-L1 low and high groups
- Higher ypN0 rates with durvalumab (58.2% vs 44.8%)
- EFS improvement seen across all levels of pathological response
The FDA-confirmed efficacy summary matched both datasets. The agency reported:
- The same EFS hazard ratio of 0.71
- The same OS hazard ratio of 0.78
- A centrally reviewed pCR rate of 19.2% with durvalumab vs 7.2% with placebo
Together, the NEJM interim publication, the ESMO 2025 final analysis, and the FDA review present a coherent and compelling picture: durvalumab + FLOT enhances tumor clearance, reduces recurrence risk, and improves survival, setting a new perioperative standard for resectable gastric and GEJ adenocarcinoma.
Safety Profile
The safety of durvalumab in combination with perioperative chemotherapy was consistent with prior studies of immune checkpoint inhibitors and FLOT-based regimens.
The prescribing information highlights risks for:
- Immune-mediated adverse events
- Infusion-related reactions
- Complications in patients with prior allogeneic stem cell transplant
- Embryo-fetal toxicity
No new safety signals were identified. Toxicities were manageable with established algorithms for immune-related events.
Recommended Dosing
For patients ≥30 kg:
- Durvalumab 1500 mg every 4 weeks with FLOT for up to 4 neoadjuvant and adjuvant cycles
- Followed by 1500 mg every 4 weeks as monotherapy for up to 10 additional cycles
For patients <30 kg:
- 20 mg/kg every 4 weeks with chemotherapy, then as monotherapy
Treatment continues until recurrence, progression, intolerable toxicity, or completion of 12 total post-operative cycles.
Find full information about approval on FDA Official Website
Conclusion
The FDA approval of durvalumab + FLOT is grounded in robust evidence demonstrating improvements in recurrence risk, tumor clearance, and early survival outcomes. These findings reinforce the value of integrating immunotherapy into perioperative treatment for resectable gastric and GEJ adenocarcinoma and support a more effective approach to curative-intent management.

Read more about MATTERHORN Trial at ESMO 2025: Durvalumab Plus FLOT in Resectable Gastric and GEJ Adenocarcinoma on OncoDaily.