Trastuzumab deruxtecan (T-DXd; Enhertu) has had its supplemental Biologics License Application (sBLA) accepted with FDA Priority Review for use in combination with pertuzumab as first-line treatment for adults with unresectable or metastatic HER2-positive breast cancer. The filing is under Real-Time Oncology Review and follows Breakthrough Therapy designation. It is supported by DESTINY-Breast09, where T-DXd plus pertuzumab reduced the risk of progression or death by 44% versus THP and achieved median PFS of 40.7 months. The FDA decision is expected in Q1 2026.

“The DESTINY-Breast09 trial showed that treating patients with HER2-positive metastatic breast cancer with Enhertu in combination with pertuzumab until progression in the first-line setting produced a new landmark of more than 40 months for progression-free survival and nearly doubled the number of patients with no evidence of disease on imaging. This marks the first major evolution in treatment in this first-line setting in more than a decade – a setting where a strong response is crucial, as up to one third of patients may not receive second-line therapy.”
said Susan Galbraith, Executive Vice President, Oncology Haematology R&D, AstraZeneca.

“Enhertu in combination with pertuzumab delayed disease progression for more than three years compared to around two years with current standard of care as a first-line treatment for patients with HER2-positive metastatic breast cancer. Receiving Priority Review moves us closer to offering Enhertu to patients even earlier in the metastatic treatment pathway as a potential new first-line treatment option.”
Ken Takeshita, Global Head, R&D, Daiichi Sankyo, said.
What is FDA Priority Review?
Under PDUFA (1992), the FDA created two review tracks: Standard and Priority. Priority Review directs extra FDA attention and resources to applications for drugs that could offer a significant improvement in treating, diagnosing, or preventing serious conditions. The FDA’s goal is to act on these applications within 6 months (vs 10 months for Standard).
What qualifies as “significant improvement”? Examples include clearly greater efficacy, fewer serious treatment-limiting adverse reactions, better adherence expected to improve serious outcomes, or evidence in a new subpopulation.
Sponsors may request Priority Review, but the FDA assigns the designation and notifies the applicant within 60 days of receiving the original BLA/NDA or efficacy supplement. Priority Review does not shorten clinical trial time and does not lower approval standards; the usual scientific/medical evidentiary bar still applies.
What is Breakthrough Therapy Designation?
BT is an FDA program to speed development and review of drugs for serious conditions when preliminary clinical evidence suggests the drug may offer a substantial improvement over available therapy on a clinically significant endpoint (e.g., effects on mortality, irreversible morbidity, serious symptoms, or strong surrogates/biomarkers). “Substantial” depends on the magnitude and clinical importance (including duration) of effect.
What does BT provide?
Breakthrough Therapy designation provides the full set of Fast Track benefits— including more frequent FDA interactions and rolling review—while adding a higher level of support and coordination. Sponsors receive intensive, early guidance that can start as soon as Phase 1, covering trial design, endpoint selection (including appropriate surrogate or intermediate endpoints), biomarker and patient-selection strategy, statistical planning, and CMC/manufacturing considerations so that the eventual submission is focused and efficient.
Communication is more iterative and time-sensitive than usual, with rapid feedback loops and additional meetings as needed to keep development on track. Although Breakthrough Therapy designation does not lower the evidentiary standard for approval, it often positions a program to be eligible for Priority Review and, when appropriate, Accelerated Approval if the totality of evidence and endpoint strategy support those pathways.
About Enhertu
Trastuzumab deruxtecan (Enhertu) is a HER2-targeted antibody–drug conjugate that delivers a topoisomerase-I payload to HER2-expressing tumor cells with a bystander effect. Since its first FDA approval in 2019 for metastatic HER2-positive breast cancer, indications expanded to HER2-positive gastric/GEJ (2021), HER2-low breast (2022), HER2-mutant NSCLC (2023), and a 2024 tumor-agnostic approval for unresectable/metastatic HER2-positive solid tumors.
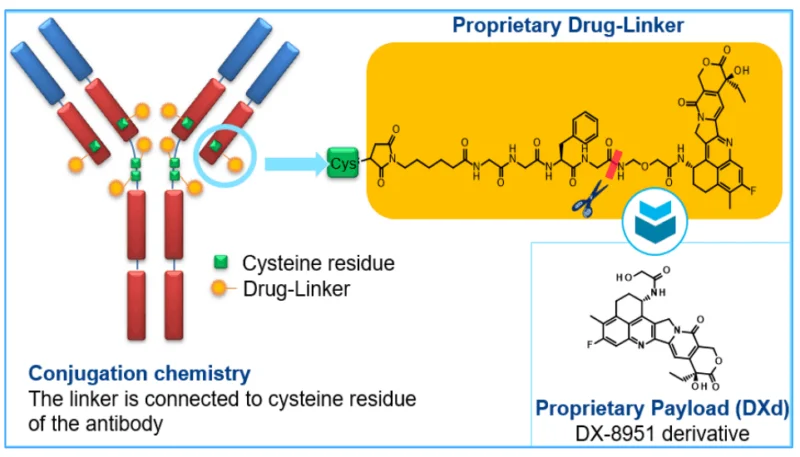
Pivotal DESTINY trials showed high response rates and survival gains across settings; ILD/pneumonitis remains the key safety risk, alongside hematologic and GI toxicities. The usual dose is 5.4 mg/kg IV q3 weeks (6.4 mg/kg for gastric/GEJ). Ongoing studies explore first-line combinations and new tumor types. Overall, Enhertu has redefined care for HER2-driven disease by extending benefit from classic HER2-positive to HER2-low and HER2-mutant cancers.
DESTINY-Breast09 Trial and what it changed.
DESTINY-Breast09 is a global, multicenter, randomized, open-label Phase III trial designed to assess the efficacy and safety of Enhertu (5.4 mg/kg) either alone or in combination with pertuzumab, compared to the standard first-line regimen of trastuzumab, pertuzumab, and a taxane (THP: docetaxel or paclitaxel) in patients with HER2-positive metastatic breast cancer.
Participants were randomized in a 1:1:1 ratio to receive either Enhertu monotherapy with a placebo for pertuzumab, Enhertu plus pertuzumab, or the standard THP regimen. Randomization was stratified based on prior treatment setting (de novo metastatic vs. progression from early-stage disease), hormone receptor (HR) status, and PIK3CA mutation status.
Primary Endpoint: Progression-free survival (PFS) by blinded independent central review.
Secondary Endpoints: Investigator-assessed PFS, overall survival (OS), objective response rate (ORR), duration of response (DoR), time to second progression, PROs, pharmacokinetics, and safety.
A total of 1,157 patients were enrolled across trial sites in Africa, Asia, Europe, North America, and South America.
The trial met its primary endpoint, showing a significant PFS benefit for the Enhertu-based regimen. Secondary endpoints, including overall survival (OS), are still being evaluated. The safety profile of the Enhertu combination was consistent with previous studies, with no new safety concerns identified.
These results suggest that the Enhertu and pertuzumab combination could become a new first-line treatment option for patients with HER2-positive metastatic breast cancer, potentially replacing the current standard of care.
For more information about trial click here.
Why does this BT designation matter?
Because it pairs compelling first-line data with the FDA’s fastest, most hands-on pathways. Breakthrough Therapy signals that early evidence suggests a substantial clinical advantage, and Priority Review—plus Real-Time Oncology Review—concentrates senior FDA resources to evaluate the sBLA within an expedited six-month clock without lowering approval standards.
Anchored by DESTINY-Breast09, where T-DXd + pertuzumab cut the risk of progression or death by 44% versus THP and achieved a median PFS of 40.7 months, this designation positions the regimen for a potential paradigm shift to a chemotherapy-free first-line option in HER2-positive metastatic breast cancer. If approved, patients could gain earlier access to a regimen with superior disease control, while sponsors and clinicians receive clearer regulatory guidance on endpoints, safety management (notably ILD monitoring), and CMC readiness to support rapid, scalable rollout.
Conclusion
In summary, FDA Priority Review for Enhertu plus pertuzumab—anchored by DESTINY-Breast09’s 44% reduction in progression or death and a median PFS of 40.7 months—signals a potential first-line shift for HER2-positive metastatic breast cancer. While Priority Review does not guarantee approval, Real-Time Oncology Review underscores the regimen’s clinical significance and could accelerate access if the benefit–risk profile holds. If authorized, clinicians may gain a potent, chemotherapy-sparing option with clear disease-control advantages, paired with vigilant ILD monitoring and established supportive care. A final decision in Q1 2026 will determine whether this combination becomes a new benchmark in the first-line setting.
You can read the full article here.


