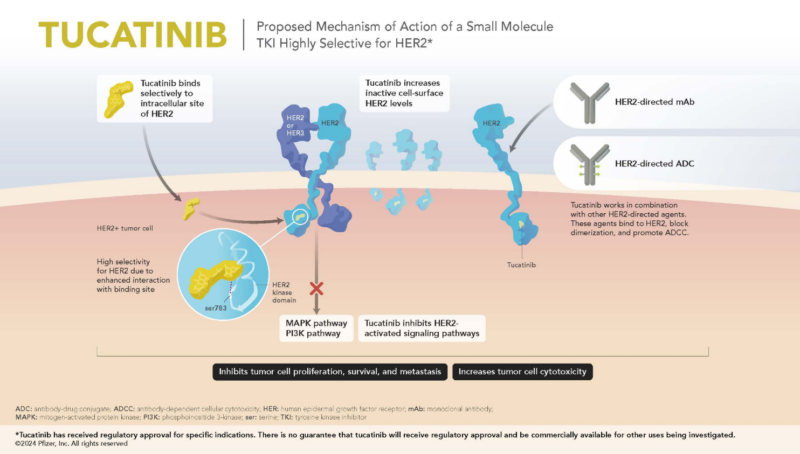
Tucatinib is an oral tyrosine kinase inhibitor (TKI) that selectively inhibits HER2, a receptor tyrosine kinase involved in the growth of certain cancers. By specifically inhibiting HER2, Tucatinib enhances the efficacy of anti-HER2 therapy while minimizing off-target effects on EGFR, reducing toxicity compared to other TKIs.
Which company produced Tucatinib?
Tucatinib (Tukysa®) was developed by Seagen Inc. (formerly Seattle Genetics), a biotechnology company specializing in targeted cancer therapies. Founded in 1997 and based in Bothell, Washington, Seagen is known for its work in antibody-drug conjugates (ADCs) and has developed FDA-approved drugs like Brentuximab Vedotin (Adcetris®) and Enfortumab Vedotin (Padcev®). In December 2023, Pfizer completed the acquisition of Seagen for approximately $43 billion, thereby expanding its oncology portfolio.
How does Tucatinib work?
Tucatinib works by selectively inhibiting the HER2 tyrosine kinase, preventing the receptor from being activated.
Normally, the HER2 receptor is part of a family of proteins involved in cell growth and survival. In HER2-positive cancers, this receptor is overexpressed, leading to continuous activation of intracellular signaling pathways like PI3K-AKT and MAPK. These pathways promote uncontrolled cell proliferation, survival, and tumor progression. In many cases, HER2 overactivation also contributes to therapy resistance and the development of metastases, including in the brain, where treatment options are often limited.
By blocking HER2 phosphorylation, Tukysa disrupts the PI3K-AKT and MAPK pathways, halting tumor cell growth and promoting cancer cell death. Unlike other HER2 inhibitors, Tucatinib is designed to avoid targeting EGFR, which helps reduce common side effects like severe diarrhea and skin toxicity.
What Cancers Is Tucatinib Approved to Treat?
Tukysa is approved for the treatment of HER2-positive metastatic or unresectable breast cancer, including cases with brain metastases, which is FDA approved in April 2020. In January 2023, Tucatinib also received approval for HER2-positive metastatic colorectal cancer (mCRC) which is RAS wild-type.

You can read more about Breast Cancer: Symptoms Causes, Stages, Diagnosis and Treatment on OncoDaily.
What research is behind the approval?
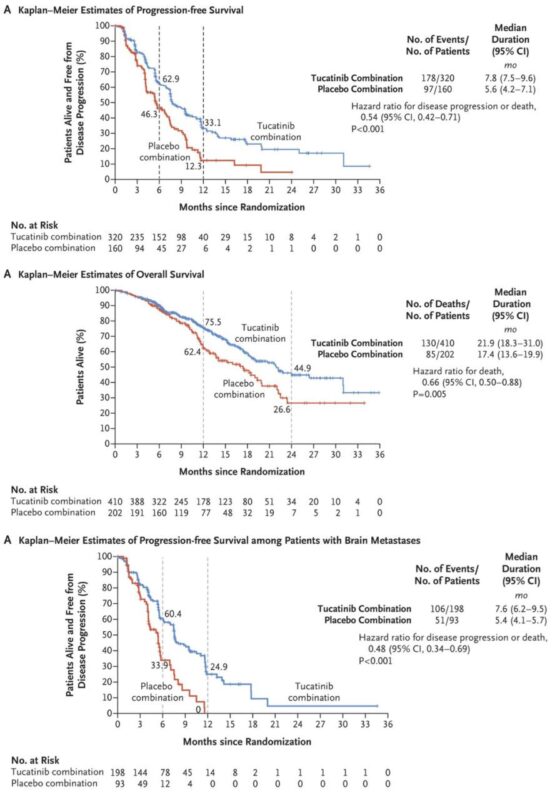
The HER2CLIMB trial, which led to Tucatinib’s FDA approval for HER2-positive metastatic breast cancer, was published in The New England Journal of Medicine on December 11, 2019. The study demonstrated that adding Tukysa to trastuzumab and capecitabine significantly improved progression-free survival (PFS) and overall survival (OS), including in patients with brain metastases. At one year, PFS was 33.1% in the Tucatinib group versus 12.3% in the placebo group, while overall survival at two years was 44.9% vs. 26.6%, respectively. Despite an increased risk of diarrhea and liver enzyme elevation, Tucatinib provided a clear survival benefit for heavily pretreated patients.
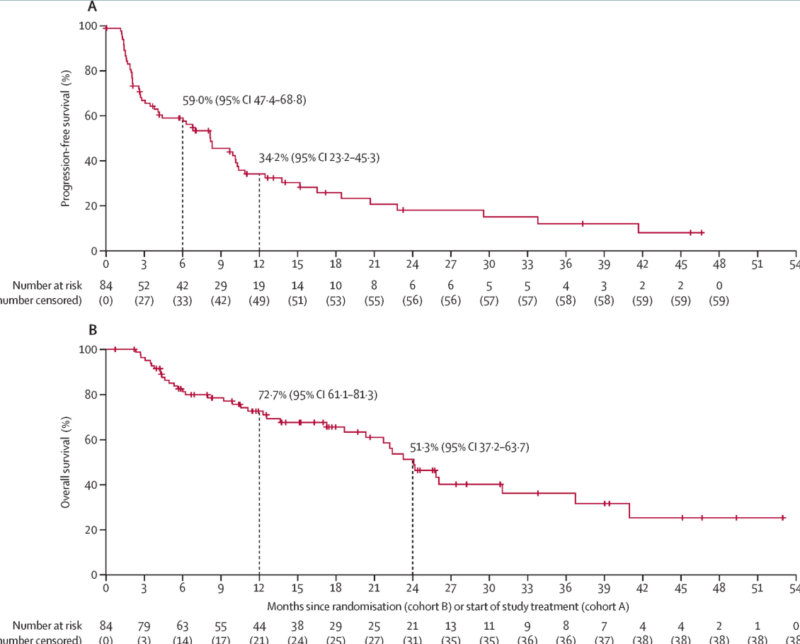
The MOUNTAINEER trial, published in The Lancet Oncology in May 2023, evaluated Tucatinib plus trastuzumab for patients with chemotherapy-refractory, HER2-positive, RAS wild-type metastatic colorectal cancer. The study enrolled 117 patients across five countries and found a confirmed objective response rate (ORR) of 38.1%, with some achieving complete responses. The combination therapy showed clinically meaningful anti-tumor activity and was well tolerated, with diarrhea and hypertension as the most common adverse events. These results led to the FDA’s accelerated approval of Tucatinib plus trastuzumab in January 2023, making it the first HER2-targeted regimen approved for metastatic colorectal cancer.
Tucatinib Combinations and Treatment Outcomes
Tucatinib, a selective HER2 inhibitor, has transformed the treatment landscape for HER2-positive cancers, particularly in combination therapies.
The HER2CLIMB trial established tucatinib with trastuzumab and capecitabine as a game-changer for metastatic HER2-positive breast cancer, especially in patients with brain metastases, significantly improving survival. Meanwhile, the MOUNTAINEER trial led to FDA approval of tucatinib plus trastuzumab for HER2-positive colorectal cancer, showing promising response rates and extended survival.
Ongoing trials explore tucatinib in novel combinations, including with trastuzumab deruxtecan (T-DXd) and immunotherapy, aiming to expand its role across HER2-driven cancers. With its proven efficacy and growing applications, tucatinib is shaping the future of HER2-targeted therapy.
Learn more about Immunotherapy for Metastatic Colon Cancer: Types, Success Rate, Side Effects on OncoDaily.
Tucatinib side effects and its management
Tukysa is a HER2 inhibitor used primarily in the treatment of HER2-positive breast and colorectal cancers. Like all medications, it can cause side effects, which vary in frequency and severity. Below is an overview of common, less common, and other side effects, along with recommended management strategies.
Common Side Effects
One of the most common side effects of tucatinib is diarrhea, which affects up to 81% of patients. In severe cases, diarrhea can lead to dehydration and kidney complications. To manage this, patients are advised to use antidiarrheal medications, stay well-hydrated, and undergo close monitoring to avoid further complications.
Another frequent issue is Hand-Foot Syndrome (Palmar-Plantar Erythrodysesthesia), which affects approximately 63% of patients. This condition manifests as redness, swelling, and pain on the palms and soles. To alleviate symptoms, patients are encouraged to use moisturizing creams, take pain relievers, and adjust their doses if necessary.
Patients may also experience nausea and vomiting, reported in about 58% and 36% of patients, respectively. Managing these symptoms typically involves administering antiemetic medications and adjusting the patient’s diet to avoid triggers.
Fatigue is another common side effect, reported in 44% of patients. Proper rest is essential, and it is important to assess for any underlying conditions, such as anemia, that might contribute to the fatigue.
Elevated liver enzymes are another concern with tucatinib. ALT levels are elevated in 46% of patients, while AST levels rise in 43%. Regular liver function tests are necessary to monitor these elevations, and dose adjustments may be required if the levels become significantly high.
Less Common Side Effects
Tucatinib can also cause less frequent side effects, such as anemia, which occurs in about 21% of patients. Monitoring blood counts is essential, and patients may require iron supplements or transfusions depending on the severity of the anemia.
Stomatitis, or mouth sores, affects 32% of patients. Good oral hygiene, along with saline rinses, can help manage discomfort associated with this side effect.
A rash is reported in 20% of patients. The rash can often be treated with topical treatments and antihistamines to reduce irritation and discomfort.
Tucatinib side effects may require dose adjustments or temporary pauses. Monitoring liver function, blood counts, and electrolytes helps detect issues early. Supportive care—hydration, nutrition, and symptom management—enhances comfort and adherence. Promptly reporting side effects ensures timely intervention and better treatment tolerance.
What is the Recommended Dosage of Tucatinib?
Tucatinib is available in 50 mg and 150 mg tablets, which is taken 300 mg twice daily. For metastatic breast cancer, it’s used with trastuzumab and capecitabine in HER2-positive cases, including brain metastases, after prior anti-HER2 treatment. In metastatic colorectal cancer, it’s combined with trastuzumab for RAS wild-type, HER2-positive tumors after progression on chemotherapy. Treatment continues until progression or intolerable side effects.
How is Tucatinib administered?
Tucatinib can be taken with or without food and should be swallowed whole, without chewing or crushing. When combined with capecitabine, both can be taken together, but capecitabine must be taken within 30 minutes after a meal. Doses should be spaced 12 hours apart at the same time each day. If a dose is missed or vomited, take the next dose as scheduled.
Store at 20-25ºC (68-77ºF) in its original container with the desiccant, protecting it from moisture. Once opened, use within 3 months, discarding any remaining tablets afterward.
What to Avoid During Tucatinib Treatment?
Avoid CYP3A-inducing or inhibiting drugs, alcohol, and grapefruit, as they can affect Tukysa’s effectiveness. Live vaccines may be less effective, and pregnancy and breastfeeding should be avoided during treatment and for one week after stopping. Limit sun exposure to reduce the risk of skin reactions. Always check with your doctor before taking new medications or supplements.
Tucatinib effectiveness over time
Published in JCO on September 26, 2023, the SGNTUC-019 phase II study evaluated tucatinib + trastuzumab in HER2-positive metastatic biliary tract cancer (mBTC). Among 30 patients, the treatment achieved a 46.7% response rate and a 5.5-month median progression-free survival. The 12-month overall survival rate was 53.6%, with pyrexia (43.3%) and diarrhea (40.0%) as the most common side effects. Grade ≥3 adverse events occurred in 60% of patients but were mostly unrelated to treatment. These findings highlight TUC+Tras as a promising option for previously treated HER2+ mBTC.
Presented at the ESMO Congress 2024 on September 14 in the poster session, this MoST program substudy evaluated tucatinib + trastuzumab in advanced solid tumors with HER2 mutations or amplification. Among 31 patients, the primary endpoint was met in the HER2 mutation group (40% response rate, median PFS 8.5 months, OS 17.2 months), while the HER2 amplification group showed limited response (6% OTR, median PFS 3.2 months, OS 16.1 months). Notably, colorectal cancer with KRAS co-mutations and HER2-mutant thyroid cancer responded, highlighting the need for further trials in these subgroups. The combination was well tolerated with no new safety concerns.
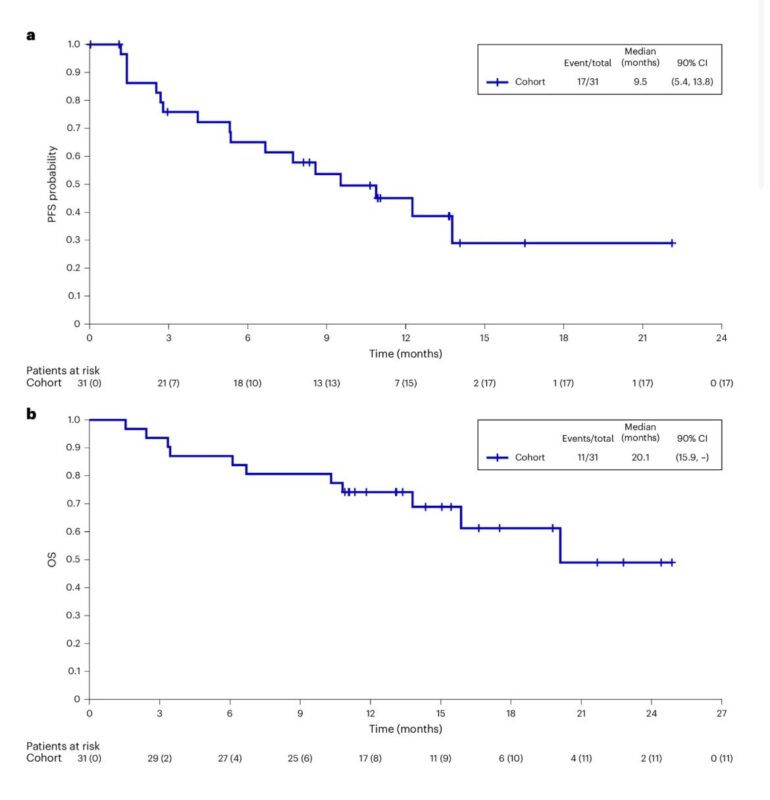
Published in Nature Medicine on January 17, 2025, this phase 2 basket trial (SGNTUC-019) evaluated tucatinib + trastuzumab in 31 heavily pretreated patients with HER2-mutated, HER2-negative metastatic breast cancer. The overall response rate was 41.9%, with a median duration of response of 12.6 months and progression-free survival of 9.5 months. Responses were seen across various HER2 mutations, and the regimen was well tolerated. These results highlight the potential of HER2-targeted therapies in HER2-mutated breast cancer, supporting further investigation.
Ongoing trials with Tucatinib
This phase I trial tests the safety, side effects, and optimal dose of Osimertinib, cetuximab, and Tukysa in EGFR-mutant NSCLC resistant to EGFR-TKI therapy. Patients receive Osimertinib (80 mg daily), Tucatinib (200 mg BID), and cetuximab (250 mg/m² IV biweekly) in 28-day cycles until progression or toxicity. The study assesses maximum tolerated dose, toxicity, and anti-tumor activity, with follow-ups every 12 weeks for up to 2 years.
The TUCATEMEB trial (NCT05673928) is a Phase II study evaluating Tukysa and ado-trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic solid tumors with brain metastases. The primary objective is to assess intracranial antitumor activity using mRECIST criteria. Key secondary objectives include measuring response using RANO-BM criteria, evaluating response duration, safety, systemic activity (RECIST v1.1), progression-free survival (PFS), and overall survival (OS). Exploratory endpoints focus on biomarkers, cfDNA dynamics, and mechanisms of resistance.
Written by Mariam Khachatryan, MD
FAQ
What is Tucatinib (Tukysa) used for?
Tucatinib is a targeted therapy used in combination with trastuzumab and capecitabine for treating HER2-positive metastatic breast cancer, including cases with brain metastases. It is also being investigated for other HER2-positive solid tumors.
For what is Tucatinib approved to treat?
Tucatinib is approved for HER2-positive metastatic breast and colorectal cancer, including cases with brain metastases.
What makes Tucatinib different from other HER2 inhibitors?
Tucatinib selectively inhibits HER2 while sparing EGFR, reducing side effects like severe diarrhea and skin toxicity that are common with other HER2-targeted tyrosine kinase inhibitors (TKIs).
What is the recommended dosage of Tucatinib?
The standard dose is 300 mg orally twice daily in combination with trastuzumab and capecitabine. It should be taken with or without food.
What are the most common side effects of Tucatinib?
Common side effects include diarrhea, fatigue, nausea, vomiting, liver enzyme elevation (AST/ALT), and hand-foot syndrome. Some patients may require dose adjustments.
How is Tucatinib stored, and what should patients know about missed doses?
Store at 20-25°C (68-77°F) in the original container. If a dose is missed or vomited, patients should take the next scheduled dose without doubling up.
What should patients expect when starting Tucatinib?
Patients should expect regular monitoring of liver function, diarrhea management strategies, and potential dose modifications to minimize side effects while maximizing effectiveness.


