Cancer treatment encompasses a range of therapeutic approaches, each with distinct mechanisms and clinical applications. The main categories include chemotherapy, which involves cytotoxic agents that target rapidly dividing cells, hormonal or endocrine therapy, which interferes with hormone signaling to control hormone-sensitive tumors, targeted therapy, which acts on specific molecular pathways critical to cancer growth and immunotherapy, which stimulates or enhances the immune system’s ability to fight cancer. Additional modalities include radiation therapy and newer strategies such as cellular and gene therapies.
Among these treatments, tamoxifen has long been a cornerstone in managing hormone receptor–positive breast cancer. However, confusion persists—both among patients and occasionally even within the clinical community—regarding whether tamoxifen should be considered a form of chemotherapy. This misconception likely arises from the drug’s systemic administration and its prominent role in cancer therapy.
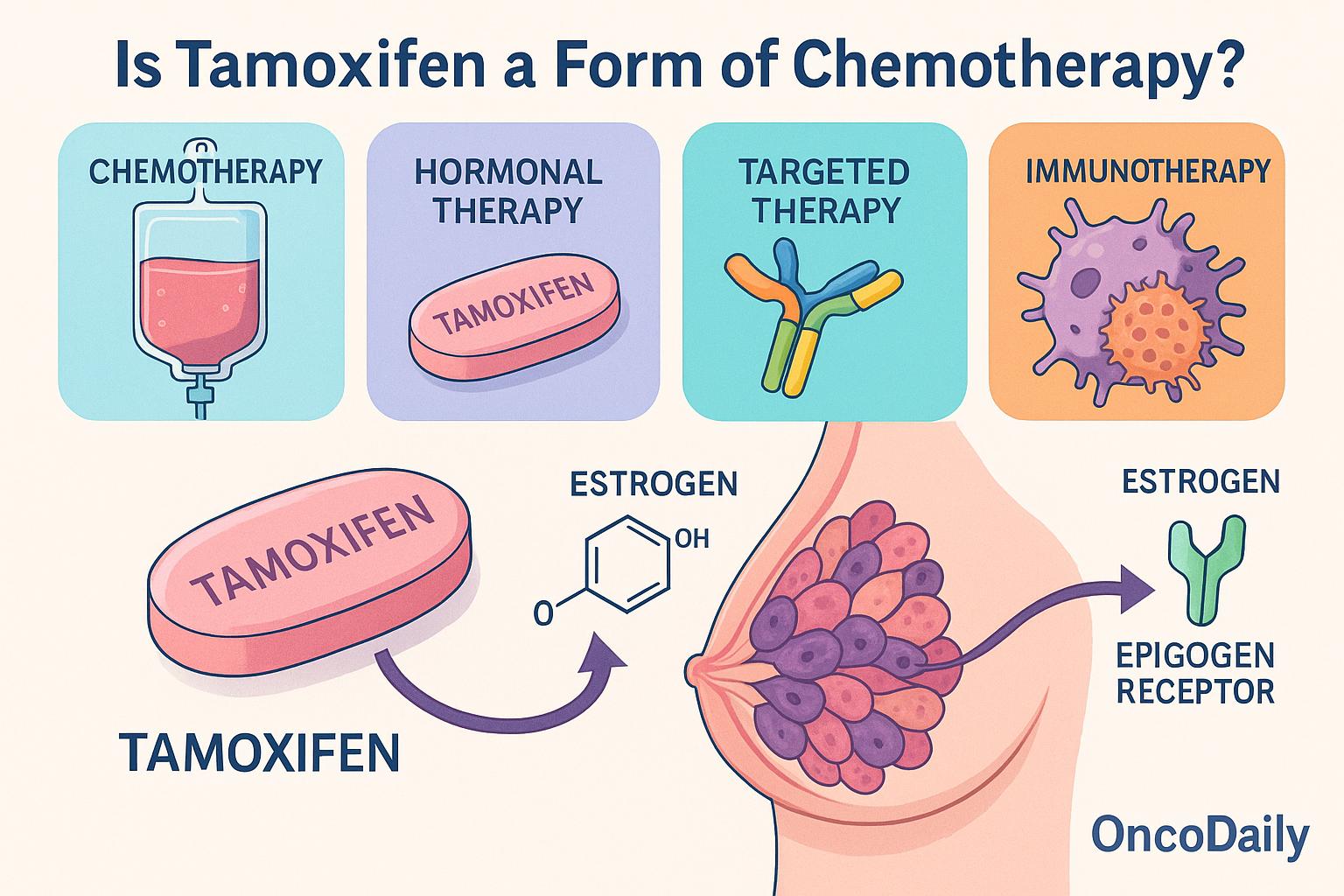
This article aims to clarify that tamoxifen is not chemotherapy, but rather a selective estrogen receptor modulator (SERM) classified under endocrine therapy. By exploring its pharmacologic profile, mechanisms of action, and therapeutic applications, this discussion will highlight the crucial distinctions between tamoxifen and cytotoxic agents, reinforcing why accurate classification is essential for treatment planning and patient education.
Tamoxifen: A Targeted Endocrine Therapy with Tissue-Specific Modulation
Tamoxifen remains a foundational agent in the treatment of estrogen receptor–positive (ER+) breast cancer, especially in premenopausal women. It is not a chemotherapy drug but rather a selective estrogen receptor modulator (SERM)—a compound that binds to estrogen receptors and modulates their activity differently in various tissues.
In breast tissue, tamoxifen acts as a competitive antagonist, preventing estrogen from binding to its receptor. This blockade inhibits the transcription of estrogen-responsive genes that drive cell proliferation, thereby suppressing tumor growth. However, in other tissues—such as the endometrium, bone, and liver—tamoxifen can function as a partial agonist, exerting weak estrogen-like effects. This dual behavior underscores both the therapeutic efficacy and side-effect profile of the drug.
Pharmacologically, tamoxifen is a prodrug. It requires metabolic activation primarily via CYP2D6 and CYP3A4enzymes in the liver to convert into its more potent active forms—4-hydroxytamoxifen and endoxifen. Endoxifen, in particular, has 30- to 100-fold greater affinity for the ER compared to the parent compound and exerts stronger anti-estrogenic effects in breast cancer cells. A 2023 review in Nature Reviews Drug Discovery by Loibl et al. highlighted the importance of CYP2D6 genotype in determining therapeutic efficacy, noting that poor metabolizers may derive less benefit from tamoxifen due to suboptimal endoxifen levels.
Additionally, transcriptomic and proteomic analyses have confirmed that tamoxifen and its metabolites alter the expression of key cell cycle regulators (e.g., cyclin D1, MYC) and apoptotic genes (e.g., BCL2), inducing growth arrest and apoptosis in ER+ cancer cells. These findings, now supported by single-cell RNA sequencing platforms, further validate tamoxifen’s mechanism of action as targeted endocrine modulation rather than generalized cytotoxicity.
From a clinical perspective, recent data from the TAILORx and MINDACT trials have reaffirmed the central role of endocrine therapies like tamoxifen in reducing recurrence risk, particularly when chemotherapy may be safely avoided in genomically low-risk, ER+ tumors.
Chemotherapy vs. Endocrine Therapy: Why Tamoxifen Is Not Chemotherapy
Chemotherapy refers to a broad class of cytotoxic agents that target rapidly dividing cells, aiming to induce cell deaththrough various mechanisms. These include direct DNA damage (as seen with alkylating agents like cyclophosphamide), inhibition of topoisomerases (e.g., anthracyclines such as doxorubicin), or disruption of mitotic spindle formation(e.g., taxanes like paclitaxel and docetaxel). The common feature of cytotoxic chemotherapy is its non-specific action against proliferating cells, leading to both antitumor effects and collateral toxicity in normal tissues, such as bone marrow, gastrointestinal mucosa, and hair follicles.
In contrast, tamoxifen is not cytotoxic. Rather, it is cytostatic, functioning to halt cell proliferation without inducing direct cell death. As a selective estrogen receptor modulator (SERM), tamoxifen modulates estrogen signaling by binding to the estrogen receptor (ER) in breast tissue, preventing estrogen-driven transcriptional activation of genes that promote tumor cell growth. Its action is receptor-specific and signaling-directed, not destructive to cellular machinery like traditional chemotherapy.
According to the NCCN Clinical Practice Guidelines in Oncology for Breast Cancer (Version 1.2025), tamoxifen is classified under the “Endocrine Therapy” section, distinct from “Systemic Chemotherapy” protocols. For example, endocrine therapy with tamoxifen is recommended for ER-positive premenopausal and postmenopausal women either as a primary adjuvant treatment or following chemotherapy, depending on clinical and genomic risk stratification.
This distinction is critical in clinical practice. While chemotherapy and endocrine therapy are often used in sequence or combination, particularly in hormone receptor–positive (HR+) tumors, they represent biologically and mechanistically separate strategies. Chemotherapy broadly targets cellular proliferation regardless of hormonal status, while tamoxifen is effective only in ER-positive cancers, highlighting its role as a targeted endocrine therapy rather than a general cytotoxic agent.
Clinical Role of Tamoxifen in Estrogen Receptor–Positive Breast Cancer
Tamoxifen remains a foundational therapy in the treatment of estrogen receptor–positive (ER+) breast cancer, particularly among premenopausal women, and continues to hold relevance in adjuvant, metastatic, and preventive settings, even as the field of endocrine therapy evolves with newer agents and genomic tools.
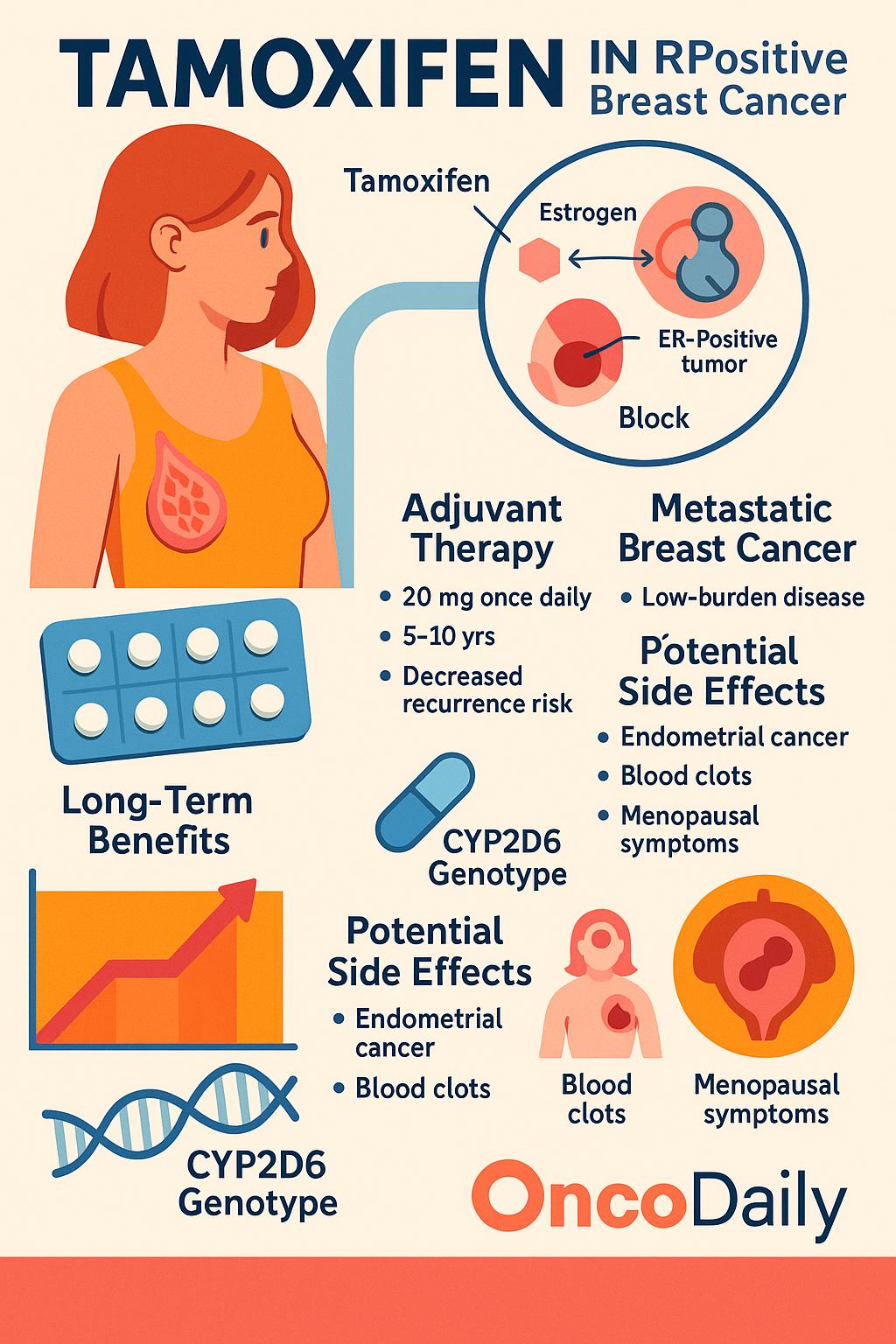
In the adjuvant setting, tamoxifen is recommended by the NCCN Breast Cancer Guidelines (Version 1.2025) for premenopausal women with ER+/HER2- tumors, either alone or in combination with ovarian suppression in higher-risk patients. The standard regimen remains 20 mg orally once daily for 5 to 10 years, with the duration tailored based on recurrence risk, nodal status, and tolerability. Newer risk stratification tools such as Oncotype DX or PAM50 (Prosigna) now help determine which patients benefit from longer endocrine therapy durations. Recent long-term analyses from the ATLAS and aTTom trials continue to support extended tamoxifen to 10 years, showing a relative reduction in breast cancer mortality by over 30% after year 10.
In metastatic ER+ disease, tamoxifen remains an effective and well-tolerated first-line option, especially for premenopausal women or those who are not candidates for aromatase inhibitors (AIs) due to comorbidities or intolerance. While CDK4/6 inhibitors in combination with endocrine therapy have become standard for many patients, tamoxifen still plays an important role in low-burden, indolent metastatic disease, where minimizing toxicity is essential. It may also serve as a bridge to more intensive regimens or when access to novel agents is limited.
In risk reduction, tamoxifen is FDA-approved for primary prevention of breast cancer in high-risk women, such as those with a Gail model risk >1.67% over 5 years, strong family history, or prior atypical hyperplasia. Updated data from the IBIS-I trial (2023 update) confirmed that the protective effect of tamoxifen persists for at least 20 years, even after completing a 5-year course, with sustained reduction in ER-positive breast cancer incidence by ~30–40%.
Recent pharmacogenomic studies have also renewed focus on CYP2D6 metabolism, as tamoxifen is a prodrug activated into endoxifen, its most potent anti-estrogen metabolite. Variants in CYP2D6 affecting metabolism (e.g., *4/*4) may impact therapeutic efficacy, although the NCCN does not currently recommend routine genotyping, citing inconsistent clinical impact. However, in specific populations with poor response or early recurrence, pharmacogenetic testing is increasingly used to guide therapy adjustments.
Importantly, tamoxifen’s safety profile remains favorable compared to chemotherapy, though endometrial cancer risk, thromboembolic events, and menopausal symptoms remain considerations. Current research is exploring lower-dose tamoxifen (e.g., 5 mg daily) as a viable strategy in select populations to retain efficacy while reducing adverse effects.
Comparison with Chemotherapy
Tamoxifen has a distinct toxicity profile compared to traditional chemotherapy. Common side effects include hot flashes, increased risk of thromboembolism, and, rarely, endometrial cancer, but it does not cause immunosuppression. In contrast, chemotherapy is often associated with alopecia, neutropenia, nausea, fatigue, and mucositis, which can significantly impact quality of life.
Tamoxifen is generally better tolerated, especially in premenopausal women with ER-positive tumors. Treatment decisions between tamoxifen and chemotherapy are guided by tumor biology, including Oncotype DX recurrence scores, lymph node involvement, and menopausal status, with endocrine therapy favored in lower-risk, hormone-sensitive disease.
Resistance Mechanisms and Current Research
While tamoxifen has transformed the management of estrogen receptor–positive (ER+) breast cancer, resistance remains a critical barrier to sustained clinical benefit. Resistance to tamoxifen can be either intrinsic or acquired and arises through several distinct mechanisms that alter estrogen signaling or activate bypass pathways.
One major mechanism is the loss of ER expression, rendering the tumor unresponsive to estrogen-blocking therapies. Another increasingly recognized cause is ESR1 mutations, particularly in the metastatic setting, which result in constitutively active estrogen receptors that no longer depend on circulating estrogen. These mutations are more common after prolonged exposure to endocrine therapy and are associated with reduced efficacy of tamoxifen.
Additionally, crosstalk between ER signaling and growth factor pathways—especially HER2 overexpression and activation of the PI3K/AKT/mTOR axis—can drive tamoxifen resistance. These pathways promote estrogen-independent proliferation and survival, thereby diminishing the drug’s effectiveness.
To address these challenges, current research is focused on combination strategies and novel agents. CDK4/6 inhibitors(such as palbociclib, ribociclib, and abemaciclib) are now widely used in combination with endocrine therapy to overcome cell cycle–mediated resistance. Another promising class includes selective estrogen receptor degraders (SERDs), such as elacestrant, which not only block but also degrade ER. The EMERALD trial demonstrated that elacestrant significantly improved progression-free survival compared to standard endocrine therapy in patients with ESR1-mutated metastatic breast cancer.
Furthermore, PI3K inhibitors like alpelisib (approved for PIK3CA-mutated HR+/HER2− breast cancer) represent a targeted approach to bypass pathway activation. Trials such as PADA-1 have explored switching endocrine therapy in response to rising ESR1 mutations detected in circulating tumor DNA, suggesting a potential for real-time monitoring and adaptive therapy.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
Is tamoxifen a type of chemotherapy?
No, tamoxifen is not chemotherapy. It is an endocrine therapy used to block estrogen receptors in ER+ breast cancer.
How does tamoxifen work in breast cancer?
Tamoxifen blocks estrogen from binding to its receptor in breast tissue, inhibiting cancer cell growth in hormone-sensitive tumors.
What are the common side effects of tamoxifen?
Hot flashes, blood clots, and endometrial changes are common. Unlike chemotherapy, it doesn't usually cause hair loss or immune suppression.
Can tamoxifen be used in premenopausal women?
Yes. According to NCCN guidelines, tamoxifen is a preferred treatment for ER+ breast cancer in premenopausal women.
What is the difference between tamoxifen and chemotherapy?
Chemotherapy destroys rapidly dividing cells. Tamoxifen is cytostatic—it blocks hormone signals without killing cells directly.
What happens if a patient is resistant to tamoxifen?
Resistance may arise from ESR1 mutations or alternative growth pathways. New treatments like CDK4/6 inhibitors and SERDs can help overcome resistance.
Is tamoxifen used in metastatic breast cancer?
Yes. It’s often used in ER+ metastatic breast cancer, especially in patients who can't tolerate or don’t require chemotherapy.
Does CYP2D6 affect tamoxifen activity?
Yes. CYP2D6 enzymes activate tamoxifen into its potent metabolite, endoxifen. Poor metabolizers may respond less effectively.
How long is tamoxifen treatment typically given?
Usually 5 to 10 years depending on recurrence risk, side effects, and clinical factors per NCCN breast cancer guidelines.
Can tamoxifen reduce the risk of breast cancer?
Yes. Tamoxifen is FDA-approved for breast cancer risk reduction in high-risk women, with lasting effects after treatment ends.