Tafasitamab-cxix is a targeted immunotherapy approved for treating certain aggressive B-cell lymphomas. Backed by the U.S. Food and Drug Administration (FDA), this monoclonal antibody has helped change the landscape of treatment for patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL). It is produced by Incyte Corporation, which acquired global rights from MorphoSys in 2024.
What Is Tafasitamab-cxix and How Does It Work?
Tafasitamab-cxix, marketed as Monjuvi, is a monoclonal antibody designed to target CD19—a protein found on the surface of B-cells. By binding to CD19, tafasitamab triggers immune system mechanisms to destroy cancerous B-cells. It enhances antibody-dependent cellular cytotoxicity (ADCC), encouraging natural killer (NK) cells to attack, and promotes antibody-dependent cellular phagocytosis (ADCP), signaling immune cells to engulf the cancer. Additionally, it can cause apoptosis, or programmed cell death, in cancer cells.
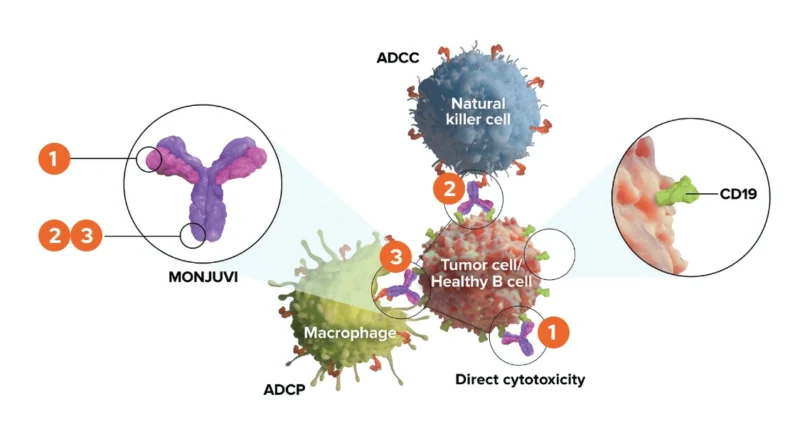
Photo from MONJUVI official website
What Cancers Does It Treat?
Tafasitamab-cxix is FDA-approved in two primary settings:
- For adults with relapsed or refractory DLBCL (not otherwise specified) who are ineligible for autologous stem cell transplant (ASCT), in combination with lenalidomide.
- For adults with relapsed or refractory follicular lymphoma (FL), in combination with lenalidomide and rituximab, as of June 2025.
Both types of lymphoma are B-cell non-Hodgkin lymphomas. DLBCL is more aggressive, while FL tends to grow more slowly but can become resistant over time.
Efficacy and Results from Clinical Trials
The FDA’s 2020 approval for DLBCL came from the L-MIND study, which showed a 55% overall response rate (ORR), including 37% complete responses. Median duration of response reached 21.7 months, highlighting long-lasting benefit.
The 2025 FL approval followed the inMIND Phase 3 trial. Adding tafasitamab to standard therapy extended median progression-free survival to 22.4 months compared to 13.9 months with placebo, reducing the risk of disease progression or death by 57% (HR 0.43; p<0.0001).
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before tafasitamab was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.

You can also read about Diffuse Large B-Cell Lymphoma: What patients should know on OncoDaily.
Tafasitamab Side Effects and Management
Tafasitamab-cxix works by helping the immune system attack cancer cells, but it can also affect healthy cells, which may cause side effects. Most patients tolerate the treatment well, but it is important to be aware of possible side effects and how they are managed.
Common Side Effects
The most common side effects involve low blood cell counts. This can lead to fatigue, higher risk of infections, and easier bruising or bleeding. Patients may also experience diarrhea, cough, fever, swelling in the hands or feet, and loss of appetite. Fatigue is especially common and may last throughout treatment.
Less Common and Serious Side Effects
Some patients might experience reactions during the infusion, especially during the first few treatments. These can include chills, flushing, rash, difficulty breathing, or high blood pressure. These reactions are usually preventable or manageable with medications given before the infusion or by adjusting the infusion speed. Other less frequent side effects include muscle or joint pain, nausea, and mild hair thinning.
Serious side effects can occur if the bone marrow is affected, causing very low white blood cell counts. This increases the risk of severe infections, which can be life-threatening in rare cases. Therefore, close medical monitoring is essential.
Managing Side Effects
Before each treatment, patients have blood tests to check for any issues. If blood counts are too low or other problems arise, the treatment may be delayed or the dose reduced to ensure safety.
Medications may be given to help increase white blood cell counts when needed. Infusion reactions are managed with premedication and supportive care. Patients are advised to practice good hygiene, avoid exposure to infections, and report any signs of illness promptly to reduce infection risks. With ongoing monitoring and appropriate care, most side effects can be controlled, allowing patients to continue their treatment safely.

How Long Does Monjuvi Stay in the Body?
Monjuvi has a half-life of about 17 days, meaning it stays active in your body for several weeks after each dose. This allows for dosing every one to two weeks.
How Is Tafasitamab-cxix Given?
Tafasitamab-cxix is given through a vein (intravenous infusion). The medicine comes as a powder that is mixed with sterile water and saline before treatment.
To lower the chance of infusion reactions, patients usually receive medicines like antihistamines or steroids before the first few infusions. If there are no reactions, these premedications may not be needed for later treatments.
The first infusion is given slowly over about 1.5 to 2.5 hours. Later infusions take about 1.5 to 2 hours. The medicine should not be mixed with other drugs in the same IV line.
Learn about Immunotherapy for Lymphoma: Types, Success Rate, Side Effects on OncoDaily.
Dosage of Tafasitamab-cxix
The dosage of tafasitamab-cxix varies depending on the type of lymphoma and is always given alongside other cancer medications. For diffuse large B-cell lymphoma (DLBCL), tafasitamab is combined with lenalidomide and given more frequently at the start, then less often over time. For follicular lymphoma (FL), tafasitamab is used with both lenalidomide and rituximab, following a similar schedule of weekly doses early on, then every two weeks.
In general, treatment cycles last several weeks, with oral medications taken daily for part of each cycle. The exact schedule depends on the patient’s condition and response to treatment, and therapy may continue until the cancer progresses or side effects become difficult to manage.
What to Expect Long-Term?
Tafasitamab isn’t a cure for most patients, but it helps control cancer effectively. In trials, it extended progression-free survival by months and, in some cases, years. If cancer progresses, doctors may recommend switching therapies or joining clinical trials.
Real-Life Effectiveness
Data from real-world use confirms the clinical trial findings. Tafasitamab continues to deliver durable responses in patients with relapsed/refractory lymphoma, even among those previously treated with multiple lines of therapy.
What Other Trials Are Ongoing?
Several ongoing trials explore tafasitamab’s potential beyond DLBCL and FL:
- TaZA CLL Study (NCT05718869): Tests tafasitamab with zanubrutinib in newly diagnosed chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
- MD Anderson Study (NCT06434363): Evaluates tafasitamab with AD-PluReceptor NK cells and chemotherapy for autoimmune diseases like lupus and systemic sclerosis.
Tafasitamab-cxix (Monjuvi) represents a valuable option for patients with hard-to-treat B-cell lymphomas. With strong clinical results, expanding research, and growing approval in multiple settings, it offers hope and new possibilities in cancer care.
If you’re a healthcare provider, access the professional version here.


