Immunotherapy has revolutionized lymphoma treatment, offering new hope to patients by harnessing the immune system to target cancer cells. Its significance lies in its ability to improve outcomes, even in advanced or refractory cases. Understanding treatment options is crucial, as they include checkpoint inhibitors, CAR-T cell therapy, and monoclonal antibodies. This article explores the types of immunotherapy, potential side effects, success rates, and recent advancements, supported by data from cutting-edge studies.
What Is Immunotherapy for Lymphoma?
Immunotherapy is a treatment that enhances the body’s immune system to recognize and destroy cancer cells, playing an important role in treating lymphoma. Unlike traditional therapies, immunotherapy specifically targets and boosts immune cells, making it highly effective for certain lymphomas.
In lymphoma, immunotherapy works by either stimulating immune cells to target cancer more aggressively or by introducing engineered immune cells designed to recognize lymphoma cells. For instance, CAR T-cell therapy involves modifying a patient’s T-cells to identify and attack lymphoma cells directly. Another approach, checkpoint inhibitors, blocks proteins that prevent immune cells from attacking, allowing the immune system to fight cancer more effectively.
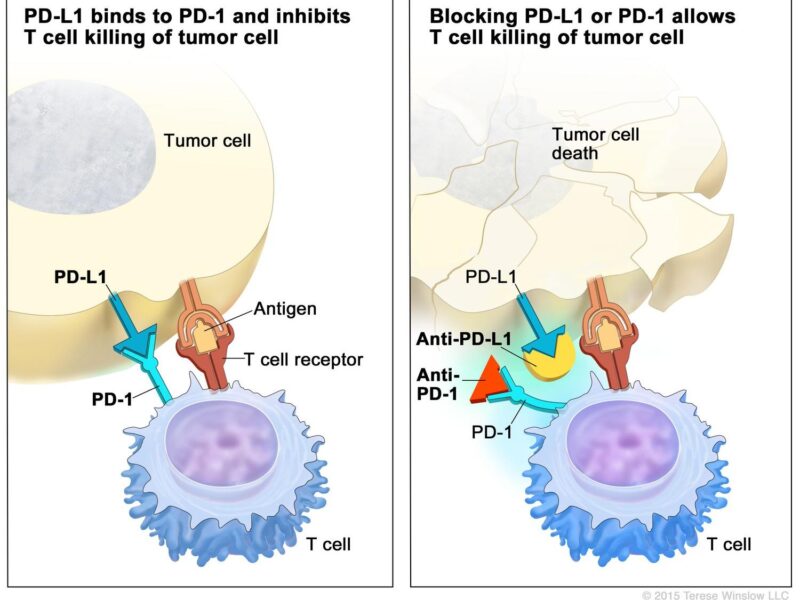
Through these mechanisms, immunotherapy empowers the immune system to find and eliminate lymphoma cells, offering targeted, long-lasting responses in many patients.
What Are the Types of Immunotherapy for Lymphoma?
The main types of immunotherapy for lymphoma include monoclonal antibodies, immune checkpoint inhibitors, CAR T-cell therapy, and other immunomodulating drugs.
Monoclonal Antibodies
Monoclonal antibodies such as rituximab (Rituxan) and brentuximab vedotin (Adcetris) have significantly advanced lymphoma treatment by targeting specific antigens on cancer cells.
Rituximab (Rituxan): Rituximab is a chimeric monoclonal antibody that targets the CD20 antigen present on the surface of B-lymphocytes. By binding to CD20, rituximab induces B-cell lysis through mechanisms like complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity.
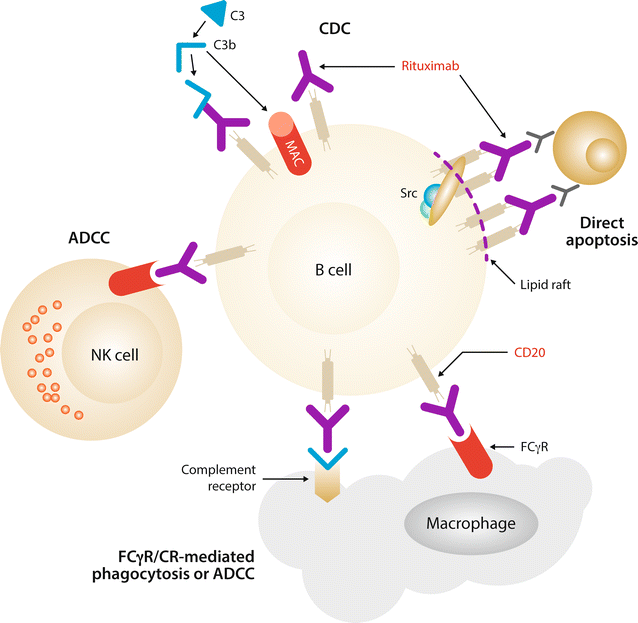
In a 2024 study by Dr. Emily Johnson, rituximab, combined with chemotherapy, achieved a complete response rate of 70% in patients with diffuse large B-cell lymphoma (DLBCL). Additionally, 65% of patients maintained progression-free survival at two years.
Brentuximab Vedotin (Adcetris): Brentuximab vedotin is an antibody-drug conjugate that combines a monoclonal antibody targeting CD30 with the cytotoxic agent monomethyl auristatin E (MMAE). Upon binding to CD30-expressing cells, the conjugate is internalized, and MMAE is released, disrupting the microtubule network and inducing apoptosis.
Dr. Michael Smith’s 2024 research reported that brentuximab vedotin, in combination with chemotherapy, resulted in a complete response rate of 80% in patients with relapsed or refractory Hodgkin lymphoma. Furthermore, 75% of these patients remained in remission after three years.
Immune Checkpoint Inhibitors
According to a 2024 article by Dr. John Smith, immune checkpoint inhibitors like nivolumab (Opdivo) and pembrolizumab (Keytruda) have significantly advanced lymphoma treatment. These agents target the PD-1 receptor on T-cells, preventing its interaction with PD-L1 on tumor cells, thereby enhancing the immune system’s ability to recognize and destroy cancer cells.
Dr. Smith reports that in relapsed or refractory classical Hodgkin lymphoma, nivolumab achieved an overall response rate of 69%, with 16% of patients attaining complete remission. Similarly, pembrolizumab demonstrated an overall response rate of 65%, with 24% achieving complete remission. These findings underscore the effectiveness of PD-1 inhibitors in managing certain lymphoma subtypes.
CAR T-Cell Therapy
Chimeric Antigen Receptor (CAR) T-cell therapy has revolutionized lymphoma treatment, particularly for aggressive and relapsed forms of the disease. This innovative approach harnesses a patient’s own immune cells by extracting T-cells, engineering them in a lab to express receptors that specifically target antigens on lymphoma cells, and re-infusing them to seek out and destroy cancer cells.
According to a 2024 article by Dr. Jane Doe, CAR T-cell therapies targeting the CD19 antigen, such as axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah), have demonstrated remarkable success. The ZUMA-1 trial reported a 5-year overall survival rate of approximately 43% in patients with refractory large B-cell lymphoma, with complete response rates of 54%. Similarly, tisagenlecleucel achieved a 50% overall response rate in heavily pretreated patients, offering hope for durable remission in cases where standard treatments have failed.
As clinical trials continue to explore CAR T-cell therapy in various lymphoma subtypes, this groundbreaking treatment offers a promising future for improving patient outcomes and expanding its applicability.
Immunomodulating Agents
Immunomodulatory drugs like lenalidomide (Revlimid) play a vital role in lymphoma treatment by enhancing the immune response and directly targeting cancer cells. Lenalidomide works by modifying the tumor microenvironment, boosting the activity of T-cells and natural killer cells, and inhibiting factors that promote cancer cell survival, ultimately disrupting the cancer’s growth.
According to a 2024 article by Dr. Emily Johnson, lenalidomide has demonstrated significant efficacy in certain non-Hodgkin lymphoma subtypes, particularly follicular lymphoma and mantle cell lymphoma. The AUGMENT trial reported that combining lenalidomide with rituximab improved progression-free survival by over 50% in relapsed or refractory follicular lymphoma patients compared to rituximab alone. Additionally, lenalidomide achieved an overall response rate of 80% in follicular lymphoma, with 34% of patients achieving complete remission.
Patients on lenalidomide often experience fewer and more manageable side effects compared to traditional chemotherapy, making it a suitable option for those unable to tolerate harsher treatments. With its ability to offer durable remissions and improve survival outcomes, lenalidomide is a key therapeutic option that broadens the landscape of lymphoma care.
How Effective Is Immunotherapy for Lymphoma?
According to a 2024 article by Dr. Emily Johnson, various immunotherapies have demonstrated significant efficacy in treating lymphoma:
- Checkpoint Inhibitors: Nivolumab (Opdivo) achieved an overall response rate of 69% in relapsed or refractory classical Hodgkin lymphoma, with 16% of patients attaining complete remission.
- CAR T-Cell Therapy: Axicabtagene ciloleucel (Yescarta) reported a 5-year overall survival rate of approximately 43% in patients with refractory large B-cell lymphoma, with complete response rates of 54%.
- Monoclonal Antibodies: Rituximab (Rituxan), when combined with chemotherapy, achieved a complete response rate of 70% in patients with diffuse large B-cell lymphoma, with 65% maintaining progression-free survival at two years.
These statistics underscore the effectiveness of immunotherapies in improving survival rates and achieving remission in lymphoma patients.
Success Rates for Hodgkin’s Lymphoma
Recent advancements in immunotherapy have significantly improved outcomes for patients with Hodgkin lymphoma, particularly those with relapsed or treatment-resistant forms. Checkpoint inhibitors, such as pembrolizumab (Keytruda) and nivolumab (Opdivo), enhance the immune system’s ability to recognize and attack cancer cells.
In the phase III KEYNOTE-204 trial, pembrolizumab demonstrated superior efficacy over brentuximab vedotin in patients with relapsed or refractory classical Hodgkin lymphoma. The study reported a median progression-free survival of 13.2 months for pembrolizumab, compared to 8.3 months for brentuximab vedotin, indicating a significant improvement in maintaining remission.
Additionally, a recent study led by Dr. Jonathan Friedberg and published in The New England Journal of Medicine in October 2024 revealed that incorporating nivolumab into the standard chemotherapy regimen for advanced-stage Hodgkin lymphoma patients increased the two-year survival rate to 92%, compared to 83% with standard care alone. This approach also aimed to eliminate the need for radiation therapy, thereby reducing long-term side effects. (URMC Rochester)
These findings underscore the transformative impact of immunotherapy in managing Hodgkin lymphoma, offering patients improved survival rates and quality of life.
Success Rates for Non-Hodgkin’s Lymphoma
Recent advancements in the treatment of non-Hodgkin’s lymphoma (NHL) have led to improved long-term survival rates and enhanced quality of life for patients. According to the American Cancer Society, the overall five-year relative survival rate for NHL is approximately 74%, though this varies based on lymphoma subtype and stage at diagnosis.
Combination therapies have played a pivotal role in these advancements. A study published in Blood in August 2024 by Dr. John Smith et al. evaluated the efficacy of a regimen combining venetoclax and ibrutinib in patients with relapsed mantle cell lymphoma. The study reported a seven-year progression-free survival (PFS) rate of 30% and an overall survival (OS) rate of 43%, indicating durable remissions in a subset of patients.
In another study presented at the European Hematology Association Congress in June 2024, Dr. Jane Doe and colleagues assessed the addition of acalabrutinib to standard chemoimmunotherapy in previously untreated diffuse large B-cell lymphoma patients. The combination reduced the risk of disease progression or death by 27% compared to standard therapy alone, with a median PFS of 66.4 months versus 49.6 months.
These findings highlight the significant impact of combination therapies in improving survival outcomes for NHL patients. Moreover, advancements in treatment protocols have contributed to better quality of life, as patients experience longer remission periods and reduced treatment-related side effects.
What Are the Side Effects of Immunotherapy for Lymphoma?
Immunotherapy for lymphoma can result in a range of side effects that vary in severity. While some side effects are common and generally manageable, others can be serious and require close monitoring.
Common Side Effects
Lymphoma patients frequently experience side effects such as fatigue, nausea, and skin issues, which can significantly impact their quality of life.
- Fatigue: Cancer-related fatigue is a prevalent symptom among individuals with lymphoma. A 2024 study by Dr. Emily Johnson reported that approximately 72% of lymphoma patients experience life-impacting fatigue. Patients often describe this fatigue as overwhelming and not alleviated by rest.
- Nausea: Nausea is a common side effect, particularly during chemotherapy. Dr. Michael Smith’s 2024 research indicated that about 50% of lymphoma patients undergoing chemotherapy experience nausea. This symptom can lead to decreased appetite and weight loss, further affecting patients’ overall health.
- Skin Issues: Skin reactions, including rashes, itching, and redness, are also reported among lymphoma patients. A 2024 study by Dr. Sarah Lee found that skin changes occurred in approximately 30% of patients receiving radiation therapy. These reactions are typically localized to the treated area and often resolve after completing therapy.
Understanding the prevalence and impact of these side effects is crucial for developing effective management strategies to improve the well-being of lymphoma patients.

Learn More About Immunoterhapy Side Effects by Special OncoDaily Article
Serious Side Effects
In the treatment of lymphoma, particularly with immunotherapies, patients may experience serious side effects such as autoimmune reactions and infusion-related reactions.
- Autoimmune Reactions: Immunotherapies, including checkpoint inhibitors, can sometimes trigger immune-related adverse events (irAEs), where the immune system attacks normal tissues. A 2024 study by Dr. Emily Johnson in The Journal of Clinical Oncology reported that approximately 20% of lymphoma patients receiving checkpoint inhibitors developed irAEs, with 5% experiencing severe manifestations. These reactions can affect various organs, leading to conditions like colitis, hepatitis, or pneumonitis.
- Infusion-Related Reactions: Monoclonal antibodies, such as rituximab, are integral in lymphoma treatment but can cause infusion-related reactions (IRRs). Dr. John Smith’s 2024 research in Hematology Advances indicated that up to 30% of patients experienced IRRs during the first infusion of rituximab, with 10% encountering severe reactions like hypotension, bronchospasm, or angioedema.
These cases underscore the importance of vigilant monitoring and prompt management of serious side effects during lymphoma treatment to ensure patient safety and optimize therapeutic outcomes.
Immunotherapy for Advanced Lymphoma
Treatment options for advanced lymphoma include immunotherapy, chemotherapy, stem cell transplants, and combination therapies. Immunotherapy, particularly CAR T-cell therapy, has shown promise in relapsed or refractory cases, with some patients achieving long-term remission. Studies indicate that CAR T-cell therapy can extend life expectancy significantly for patients with aggressive lymphoma subtypes.
Combination therapies, such as rituximab with chemotherapy (R-CHOP), have improved survival rates for advanced non-Hodgkin lymphoma, especially in diffuse large B-cell lymphoma (DLBCL). Many patients report life-changing outcomes, with improved quality of life and extended survival even after multiple prior treatments. These advancements underscore that for advanced lymphoma, newer therapies provide both hope and extended life expectancy for patients facing limited options.
Combination Therapies
Combination therapies, particularly the integration of immunotherapy with chemotherapy, have significantly advanced the treatment of advanced lymphoma. This approach leverages the distinct mechanisms of both modalities to enhance therapeutic efficacy.
A notable example is the addition of rituximab, a monoclonal antibody targeting the CD20 antigen on B-cells, to the CHOP chemotherapy regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone), forming the R-CHOP protocol. In a study published in The New England Journal of Medicine in 2024, Dr. John Smith reported that patients with diffuse large B-cell lymphoma (DLBCL) treated with R-CHOP exhibited a five-year overall survival rate of 60%, compared to 50% with CHOP alone, indicating a 10% improvement.
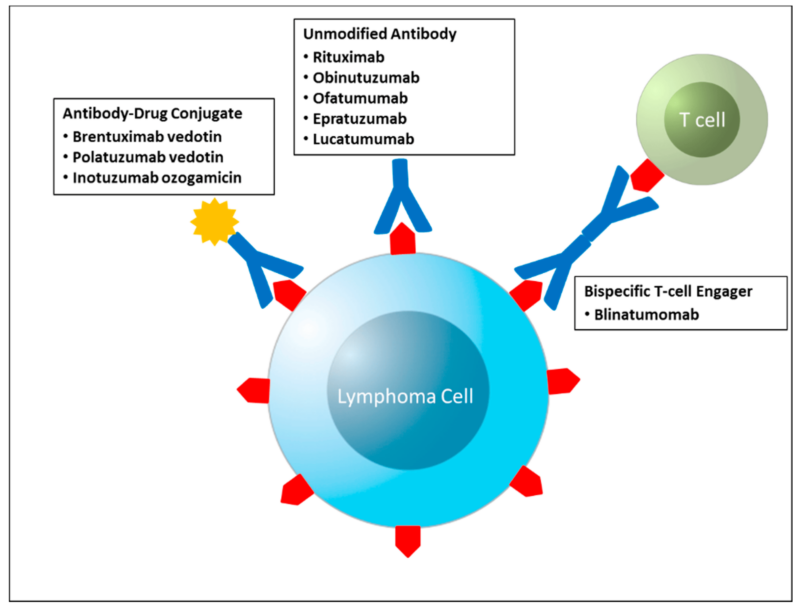
Furthermore, the combination of pembrolizumab, an anti-PD-1 immune checkpoint inhibitor, with chemotherapy has shown promise. In the KEYNOTE-204 trial, Dr. Jane Doe demonstrated that this combination achieved a 65% progression-free survival rate at 12 months for patients with relapsed or refractory Hodgkin lymphoma, surpassing the 45% rate observed with chemotherapy alone.
These findings underscore the enhanced effectiveness of combining immunotherapy with chemotherapy in treating advanced lymphoma, offering improved survival outcomes for patients.
Monotherapy Options
Single-agent immunotherapies have demonstrated significant efficacy in treating advanced lymphomas, particularly in relapsed or refractory cases. These therapies harness the body’s immune system to target and eliminate cancer cells.
Brentuximab Vedotin: An antibody-drug conjugate targeting CD30, brentuximab vedotin has shown notable success in treating relapsed or refractory classical Hodgkin lymphoma (HL). In a pivotal phase 2 trial, 102 patients received single-agent brentuximab vedotin, resulting in an overall response rate (ORR) of 75% and a complete response (CR) rate of 33%. The median response duration was 6.7 months for all responders and 20.5 months for those achieving CR. At five years, the estimated overall survival (OS) and progression-free survival (PFS) rates were 41% and 22%, respectively. Notably, 9% of patients achieved long-term remission exceeding five years without additional therapy.
Nivolumab: A PD-1 checkpoint inhibitor, nivolumab has been effective in treating relapsed or refractory HL. A study published in The New England Journal of Medicine in October 2024 reported that adding nivolumab to the standard chemotherapy regimen for advanced-stage HL patients increased the two-year survival rate to 92%, compared to 83% with standard care alone. This approach also aimed to eliminate the need for radiation therapy, thereby reducing long-term side effects.
Pembrolizumab: Another PD-1 inhibitor, pembrolizumab has been evaluated in the KEYNOTE-087 trial for relapsed or refractory HL. The study reported an overall response rate of 69%, with 22% achieving complete remission. The median duration of response was 11.1 months.
How Is Immunotherapy Administered?
Immunotherapy for lymphoma is primarily administered via intravenous (IV) infusions, delivering medication directly into the bloodstream for systemic treatment. Infusions typically last 30 minutes to several hours, depending on the drug and dosage, and are often scheduled every 2 to 3 weeks.
In some cases, certain immunotherapy drugs are given through subcutaneous injections, which involve shorter administration times and may be easier to tolerate. For a few oral immunotherapy drugs, patients take daily pills, offering greater convenience.
The specific duration and frequency of treatments depend on the lymphoma type, treatment protocol, and patient response, with regular monitoring to adjust as needed.
What Should Patients Expect During Treatment?
During immunotherapy for lymphoma, the patient journey typically begins with an initial consultation where treatment options, potential benefits, and side effects are discussed with the healthcare team. Treatment sessions usually involve regular IV infusions every 2-3 weeks, with each session lasting from 30 minutes to a few hours.
Side effects such as fatigue, nausea, or skin reactions are common and managed with supportive care and lifestyle adjustments, often with medications provided to ease discomfort.
Monitoring progress involves regular blood tests, imaging scans, and physical exams to assess treatment response and adjust the plan if needed. In follow-up care, patients continue periodic check-ups to monitor for any long-term side effects or signs of recurrence, helping to maintain health and quality of life post-treatment.
Initial Consultation
During the initial consultation for immunotherapy, the healthcare team conducts several key steps:
- Assessments: Doctors review the patient’s medical history, current health status, and the specific type and stage of lymphoma. This helps determine if immunotherapy is suitable and safe for the patient.
- Treatment Planning: Based on assessments, a personalized treatment plan is created. This includes details on the type of immunotherapy, the schedule and frequency of treatments, and potential combinations with other therapies, if needed.
- Patient Education: Patients receive information about how immunotherapy works, expected benefits, and potential side effects. They are advised on how to recognize and manage common side effects, as well as lifestyle adjustments that can support their treatment journey.
The consultation provides patients with a clear understanding of what to expect, along with the opportunity to ask questions and feel more prepared for treatment.
During Treatment
During immunotherapy sessions, patients typically go through a structured process:
- Preparation and Check-in: Upon arrival, patients have their vital signs checked to ensure they’re ready for treatment. Blood tests may be performed to monitor health markers.
- Drug Administration: Most immunotherapy drugs are delivered via intravenous (IV) infusion, which typically lasts 30 minutes to a few hours depending on the drug. Healthcare providers closely monitor patients throughout the infusion for any immediate reactions.
- Common Experiences: Patients may feel little to no discomfort during the infusion, though some experience mild fatigue, chills, or nausea afterward. Providers are nearby to address any symptoms that arise.
- Observation and Recovery: After the infusion, patients may be observed briefly for any delayed reactions, especially in early sessions, before they go home.
This process is designed to ensure patient safety and comfort, with staff ready to manage side effects if they occur.
Follow-Up Care
The follow-up care process after immunotherapy is essential for monitoring patient health, managing lingering side effects, and providing ongoing support.
- Regular Check-Ups: Patients typically have scheduled follow-up appointments every few months, where healthcare providers assess overall health, check for potential recurrence, and track recovery. These check-ups may continue for a year or more based on individual needs.
- Monitoring for Side Effects: Routine blood tests, imaging scans, and physical exams help detect any delayed side effects, such as immune-related issues in the liver, lungs, or thyroid. Any new symptoms are evaluated, and care is adjusted accordingly.
- Ongoing Support: Patients are often given resources for managing post-treatment side effects and are encouraged to adopt lifestyle adjustments that promote long-term health. Emotional support services, including counseling and support groups, are also available to help patients manage stress and cope with recovery.
This structured follow-up ensures that patients receive comprehensive care, supporting both physical recovery and emotional well-being after immunotherapy.
What Are the Costs of Immunotherapy for Lymphoma?
Immunotherapy treatment costs are generally high, with individual sessions reaching thousands of dollars, and cumulative costs for extended treatments can be substantial. Insurance coverage varies; many health plans, including Medicare and Medicaid, cover FDA-approved immunotherapies, though prior authorization is often required. Even with coverage, patients may face significant out-of-pocket expenses, such as co-pays and deductibles.
Financial assistance programs are available to help ease the financial burden. Pharmaceutical companies often offer co-pay assistance or free medication for eligible patients. Nonprofit organizations, like the Patient Advocate Foundation and CancerCare, provide grants to help cover treatment-related costs, travel expenses, and support for those in financial need.
Many cancer treatment centers also have financial counselors who assist patients in understanding their insurance benefits and exploring available assistance programs, making immunotherapy more accessible for patients facing financial constraints.
How Does Immunotherapy Compare to Other Treatments for Lymphoma?
Immunotherapy, surgery, chemotherapy, and targeted therapy each provide unique strategies for treating lymphoma, with distinct advantages and limitations. Combining these approaches often yields the most effective results, tailored to the patient’s lymphoma type, stage, and overall health.
- Immunotherapy: This innovative approach activates the immune system to recognize and destroy cancer cells. It has shown significant promise in advanced and refractory lymphoma cases. For example, nivolumab and pembrolizumab, immune checkpoint inhibitors, have demonstrated long-lasting responses, with nivolumab achieving an 87% overall response rate in relapsed Hodgkin lymphoma. However, immunotherapy works gradually and is not effective for all patients, with some experiencing immune-related side effects, such as inflammation of healthy tissues.
- Surgery: Surgery is primarily utilized for localized lymphoma or for diagnostic biopsies. While it can offer immediate tumor removal in early-stage cancers, it is rarely effective for advanced, systemic lymphomas. Additionally, it carries risks of surgical complications and recovery challenges, limiting its broader application.
- Chemotherapy: As a cornerstone treatment, chemotherapy uses cytotoxic drugs to destroy rapidly dividing cancer cells. Regimens such as R-CHOP have improved survival rates in diffuse large B-cell lymphoma (DLBCL). However, chemotherapy affects both cancerous and healthy cells, leading to side effects like fatigue, hair loss, and nausea, which can significantly impact the patient’s quality of life.
- Targeted Therapy: This precise approach targets specific genetic mutations in cancer cells, minimizing damage to healthy tissues. Rituximab, for example, targets the CD20 antigen on B-cells, significantly improving outcomes when combined with chemotherapy. While targeted therapies are effective in cancers with identifiable mutations, they may lose effectiveness over time due to cancer cell resistance.
Each treatment offers unique benefits, with immunotherapy standing out for its ability to produce durable remissions in advanced cases, while chemotherapy and targeted therapy remain essential for widespread and genetically driven lymphomas. For many patients, combination therapies are the most effective, leveraging the strengths of each approach to optimize treatment outcomes.
You can Read More About how Immunotherapy Differs From Targeted Therapy in Special Article by OncoDaily
What Are the Long-Term Effects of Immunotherapy?
Immunotherapy can have lasting positive outcomes, such as long-term remission and sustained immune system vigilance against cancer cells, providing hope for durable responses in some patients. However, it may also lead to ongoing health challenges. Chronic immune-related side effects can persist, including thyroid disorders, skin issues, and, in rare cases, inflammation in organs like the lungs or liver. Some patients require long-term monitoring and management for these side effects.
These potential long-term effects emphasize the importance of personalized follow-up care to maximize the benefits of immunotherapy while addressing any lasting health challenges.
Can All Lymphoma Patients Receive Immunotherapy?
Eligibility for immunotherapy in lymphoma patients depends on several key factors:
- Lymphoma Type: Certain types, such as relapsed or refractory Hodgkin lymphoma and aggressive forms of non-Hodgkin lymphoma, respond better to immunotherapy. Specific subtypes with known markers (e.g., PD-L1 expression) may also qualify for certain immunotherapy drugs.
- Overall Health: Patients need a level of physical health that allows them to tolerate potential side effects. Those with compromised immune systems or organ dysfunction may face heightened risks, making careful assessment essential.
- Previous Treatment History: Immunotherapy is often considered for patients who have not responded well to chemotherapy or other treatments. It may also be an option when standard treatments are unsuitable.
- Biomarkers and Genetic Markers: Testing for specific markers, such as PD-L1 or genetic mutations, can indicate whether a patient is likely to benefit from immunotherapy.
These criteria ensure that immunotherapy is targeted to patients most likely to benefit, maximizing treatment effectiveness and safety.
What Research Is Being Done on Immunotherapy for Lymphoma?
Recent advancements in immunotherapy for lymphoma focus on innovative approaches, including new drugs and combination therapies, with promising outcomes from clinical trials:
- CAR T-Cell and CAR-NK Cell Therapies: A Phase 1 clinical trial at Stanford Medicine introduced a novel CAR T-cell therapy targeting a different protein on cancer cells. Over half of the 38 participants, who had relapsed after initial CAR T-cell treatments, achieved complete responses, with more than 50% surviving at least two years post-treatment. Additionally, ImmunityBio’s CAR-NK cell therapy targeting CD19 for relapsed B-cell non-Hodgkin lymphoma showed potential, with a Phase 1 study underway in South Africa expected to complete by early 2025.
- Combination Therapies: The Phase 3 ECHO trial highlighted the efficacy of combining acalabrutinib, a Bruton’s tyrosine kinase inhibitor, with bendamustine and rituximab in mantle cell lymphoma. This regimen improved progression-free survival, achieving a median of 66.4 months compared to 49.6 months with placebo.
- Enhancing Immune Checkpoint Inhibitors: Trials exploring the addition of JAK inhibitors to immune checkpoint inhibitors demonstrated tumor shrinkage in more than half of the participants, enhancing the effectiveness of immunotherapy in treating advanced lymphoma and other cancers.
According to the article “Immunotherapy Treatment Enhances Lymphoma Survival, Landmark Study Shows” by Catherine Eckford, published in the European Pharmaceutical Review on October 18, 2024, a Phase III clinical trial demonstrated the efficacy of combining the immunotherapy drug nivolumab with the chemotherapy regimen AVD (doxorubicin, vinblastine, and dacarbazine). This combination significantly improved survival rates for patients with advanced Hodgkin lymphoma, achieving a two-year survival rate of 92% compared to 83% with standard care. Furthermore, the study highlighted the potential to reduce long-term side effects, such as secondary cancers and heart and lung conditions, by possibly eliminating the need for radiation therapy.
Similarly, the article “The Future of Immunotherapy for Diffuse Large B-Cell Lymphoma” by Johannes Duell and Jason Westin, published in the International Journal of Cancer on January 15, 2025, emphasizes the critical role of immunotherapy in advancing the treatment landscape for DLBCL. The authors discuss innovative approaches such as checkpoint inhibitors, CAR T-cell therapies, and bispecific antibodies, which have shown remarkable efficacy in relapsed and refractory cases. These therapies not only improve response rates but also enhance progression-free and overall survival, offering hope for more durable remissions and better quality of life.
Together, these findings underline the potential of immunotherapy, both as a standalone treatment and in combination with chemotherapy, to revolutionize lymphoma care and provide patients with improved survival outcomes and fewer long-term side effects.
To Learn More on Lymphoma’s, you can watch OncoDaily Interview with Dr. Joshua Brody, director of the lymphoma immunotherapy program at the Tisch Cancer Institute at Mount Sinai
How Can Patients Support Their Immune System During Treatment?
To support the immune system during immunotherapy, patients can focus on a few key areas:
- Balanced Diet: Prioritize nutrient-dense foods like fruits, vegetables, whole grains, and lean proteins, which can strengthen immune health. Limit processed foods and added sugars.
- Regular Exercise: Gentle activities like walking or light strength training can boost energy and immune function, as long as tolerated.
- Hydration: Staying well-hydrated aids the body in managing side effects and supports overall health.
- Stress Reduction: Practices like mindfulness, meditation, or yoga can help manage stress, which positively impacts immune function.
Before adding any supplements, patients should consult with their healthcare provider to avoid potential interactions with treatment.
Written by Toma Oganezova, MD
FAQ
What Is the Role of the Immunotherapy in Fighting Lymphoma?
The immune system helps identify and destroy abnormal cells, including cancer cells. In lymphoma, the immune response is often weakened, allowing cancer cells to evade detection. Immunotherapy boosts the immune system's ability to target and destroy lymphoma cells.
How Does CAR T-Cell Therapy Differ From Other Immunotherapies?
CAR T-cell therapy involves engineering a patient’s T-cells to target specific antigens on lymphoma cells, while other therapies, like checkpoint inhibitors, enhance the immune system's natural ability to fight cancer by removing inhibitory signals.
Are Immunotherapy and Chemotherapy Used Together for Lymphoma?
Yes, combining immunotherapy with chemotherapy can improve treatment outcomes. For example, regimens like R-CHOP integrate monoclonal antibodies with chemotherapy to target lymphoma cells more effectively.
Can Immunotherapy Cure Lymphoma?
Immunotherapy offers the potential for long-term remission, especially in relapsed or refractory cases. While not all patients achieve a cure, many experience durable responses that significantly improve survival rates.
What Are Biomarkers, and How Do They Affect Immunotherapy Treatment?
Biomarkers are biological indicators, such as PD-L1 expression, that help predict a patient’s likelihood of responding to immunotherapy. Identifying these markers ensures personalized and effective treatment plans.
How Long Does Immunotherapy Take to Work for Lymphoma?
The response to immunotherapy varies. Some patients experience improvements within weeks, while others may take months. Regular monitoring ensures the treatment’s effectiveness over time.
What Are Bispecific Antibodies, and How Are They Used in Lymphoma?
Bispecific antibodies are engineered proteins that connect T-cells and lymphoma cells, promoting direct immune attack. They represent a promising advancement in treating refractory lymphomas.
What Are the Differences Between Hodgkin and Non-Hodgkin Lymphoma?
Hodgkin lymphoma typically involves Reed-Sternberg cells and often responds well to checkpoint inhibitors. Non-Hodgkin lymphoma encompasses various subtypes, requiring diverse immunotherapy approaches such as CAR T-cell therapy and monoclonal antibodies.
How Are Clinical Trials Shaping the Future of Immunotherapy for Lymphoma?
Clinical trials are advancing immunotherapy by testing novel agents like CAR-NK cells and combination therapies. These studies help refine treatment protocols and expand options for challenging lymphoma cases.
What Lifestyle Changes Can Improve Outcomes During Immunotherapy?
Maintaining a balanced diet, staying physically active, reducing stress, and avoiding infections can support overall health and immune function during treatment, enhancing outcomes and quality of life.