Sacituzumab govitecan, marketed as Trodelvy, is an innovative cancer therapy known as an antibody–drug conjugate (ADC). By combining a targeted monoclonal antibody with a potent chemotherapy agent, Trodelvy delivers cancer-killing effects directly to tumor cells while minimizing damage to healthy tissue. The drug has FDA approval for several difficult-to-treat cancers, including metastatic triple-negative breast cancer (TNBC) and hormone receptor–positive, HER2-negative (HR+/HER2–) breast cancer, providing new options for patients whose disease has progressed after standard treatments.
What Is Trodelvy and How Does It Work?
Trodelvy is designed to exploit a protein called Trop-2, which is present at high levels on the surface of many aggressive cancer cells. Trop-2 is involved in tumor growth and spread, making it an ideal target for therapy. The antibody component of Trodelvy binds specifically to Trop-2, carrying its chemotherapy payload, SN-38, into the cancer cell. Once inside, SN-38 interferes with DNA replication and repair, ultimately causing the cancer cell to die.
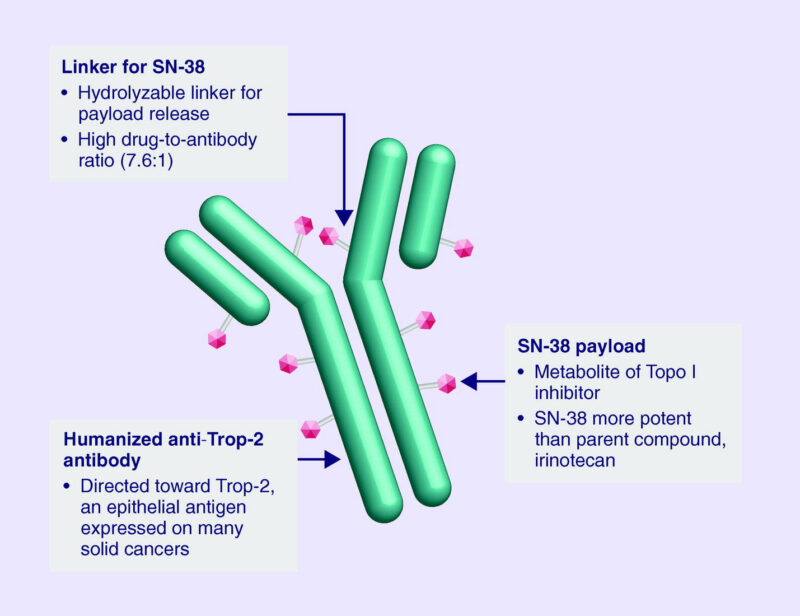
A unique feature of Sacituzumab Govitecan is that its drug-linker system can release SN-38 not only within the targeted cancer cell but also into the surrounding tumor environment. This bystander effect allows the drug to attack neighboring cancer cells that may express lower levels of Trop-2, increasing its overall effectiveness. By concentrating the chemotherapy where it is needed most, Trodelvy enhances tumor-killing activity while aiming to reduce systemic side effects.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Sacituzumab Govitecan was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.
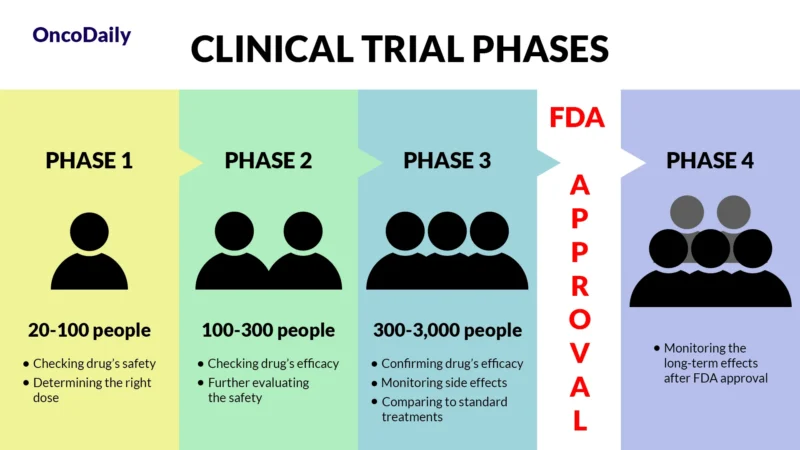
What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
What Cancers Does Trodelvy Treat?
Trodelvy’s FDA approvals reflect its role in treating aggressive cancers with limited treatment options:
- Metastatic triple-negative breast cancer (TNBC): For adults with unresectable or metastatic disease who have received at least two prior therapies.
- Hormone receptor–positive, HER2-negative breast cancer: For adults whose disease has progressed after endocrine therapy and at least two systemic treatments.
Sacituzumab Govitecan was previously approved for locally advanced or metastatic urothelial carcinoma, but this indication was withdrawn in November 2024 after trials did not show a survival benefit.
Evidence from Clinical Trials
Trodelvy’s approvals are supported by clinical studies demonstrating meaningful patient benefits.
In metastatic TNBC, the Phase 3 ASCENT trial enrolled 529 patients with disease progression after two or more prior therapies. Sacituzumab Govitecan extended median progression-free survival to 4.8 months versus 1.7 months for chemotherapy, and overall survival to 11.8 months versus 6.9 months. Side effects were generally manageable and included nausea, diarrhea, fatigue, neutropenia, and hair loss.
For metastatic urothelial carcinoma, the Phase II TROPHY-U-01 trial enrolled 113 patients who had progressed after platinum-based chemotherapy and immunotherapy. Trodelvy achieved a 27% objective response rate, with 77% of patients experiencing tumor shrinkage. Median progression-free survival was 5.4 months, and overall survival was 10.9 months.
In HR-positive, HER2-negative breast cancer, the Phase 3 TROPiCS-02 trial included 543 patients. Sacituzumab Govitecan increased median progression-free survival to 5.5 months and overall survival to 14.4 months, compared with 4 months and 11.2 months, respectively, for standard chemotherapy. Common side effects were low blood counts, diarrhea, fatigue, nausea, hair loss, and elevated blood glucose.
Real-world data from June 2025 confirmed similar outcomes in patients with metastatic TNBC, supporting both effectiveness and manageable safety in clinical practice.
How Sacituzumab Govitecan Is Administered?
Trodelvy is administered as an intravenous infusion in a 21-day cycle, with treatments given on Day 1 and Day 8 of each cycle. The first infusion typically takes about three hours, allowing medical teams to observe the patient and respond to any infusion-related reactions. If the initial infusion is well tolerated, subsequent treatments may be delivered over a shorter period, usually one to two hours.
Patients receive premedications before each infusion to reduce the risk of nausea and allergic reactions. These may include anti-nausea medicines, antihistamines, and acid-reducing drugs. Some patients may also receive corticosteroids, especially if they have experienced infusion reactions during earlier cycles. Because neutropenia is common with Trodelvy, preventive use of growth-factor support such as G-CSF is sometimes recommended, particularly for patients at higher risk. Treatment continues as long as the cancer remains controlled and the side-effects are manageable.
Trodelvy Side Effects and Management
Trodelvy carries side effects due to its chemotherapy component, but many can be anticipated and managed.
Common side effects
Side effects are a natural part of most cancer treatments, and Trodelvy is no exception. The most common side effect is neutropenia, a decrease in white blood cells that can make patients more vulnerable to infection. Regular blood tests are performed to monitor cell counts. When needed, medications like G-CSF help restore the immune system more quickly. Diarrhea is another frequent side effect, and early management with anti-diarrheal medications and hydration is essential for preventing complications.
Other side effects may include nausea, vomiting, fatigue, hair loss, mouth sores, and anemia. These effects are familiar to many patients who have previously received chemotherapy. With Trodelvy, supportive care remains a crucial component of treatment. Most side effects can be managed through medications, dietary adjustments, hydration, and, when necessary, temporary treatment delays or dose reductions.

Less common side effects
Less common, serious side effects can occur, such as febrile neutropenia, dehydration, liver enzyme changes, or infusion-related reactions. Providers typically monitor for these issues throughout treatment, and patients are encouraged to communicate openly about symptoms so that adjustments can be made promptly. With close monitoring and early management, many individuals remain on Trodelvy long enough to experience meaningful benefit.
Ongoing and Emerging Research
Research into sacituzumab govitecan continues to expand, exploring its potential in additional cancer types and combination strategies. One active area of investigation focuses on esophageal squamous cell carcinoma (ESCC), where Trop-2 is frequently overexpressed. A Phase II trial at National Taiwan University Hospital is studying Trodelvy in patients whose tumors show high Trop-2 expression, aiming to determine whether the drug can offer benefit in this difficult cancer type.
Trodelvy is also being evaluated in platinum-resistant ovarian, fallopian tube, and peritoneal cancers through a Phase II study at Yale University. These cancers often recur after treatment and are challenging to manage, creating interest in targeted therapies like Trodelvy that deliver concentrated chemotherapy to tumor cells.
In addition, researchers are studying Trodelvy in combination with immunotherapy. Trials combining Trodelvy with pembrolizumab (Keytruda®) explore whether ADC-induced tumor cell death can stimulate the immune system and improve responses. These combination studies may help determine whether Trodelvy has a role earlier in the treatment course for select cancers.
What Patients Can Expect?
During treatment, patients typically receive outpatient infusions and regular monitoring of blood counts and organ function. Supportive care is essential for managing side effects, maintaining quality of life, and continuing therapy safely. While Trodelvy is not curative, it can control disease, shrink tumors, and extend survival, providing valuable treatment time and options for patients with challenging cancers.
Looking Ahead
Trodelvy represents a major advancement in ADC technology and in the treatment of metastatic breast cancer. As research continues, its potential may expand into new cancer types and treatment combinations. Trodelvy has already reshaped expectations for patients facing aggressive metastatic disease, offering an option that can extend survival and improve quality of life. With ongoing clinical trials, scientific interest in Trop-2 targeting, and promising results in combination therapies, the role of sacituzumab govitecan in modern oncology is likely to grow.


