Repotrectinib is a next-generation targeted therapy developed to treat certain advanced cancers driven by specific genetic mutations. Unlike traditional chemotherapy, Repotrectinib works by blocking abnormal proteins that help cancer cells grow and spread. Its precision-targeted approach offers new hope for patients, especially those who have already received prior treatments or whose tumors have become resistant to earlier therapies. This article will help you explore its uses in cancer, possible side effects, recommended dosage, what to expect during treatment, and more.
Which company produced Repotrectinib?
Repotrectinib, marketed under the brand name Augtyro, was originally developed by Turning Point Therapeutics, Inc., a biotechnology company focused on creating precision medicines for cancer patients with specific genetic alterations. In 2022, Turning Point Therapeutics was acquired by Bristol‑Myers Squibb (BMS), one of the world’s largest pharmaceutical companies. Since then, BMS has taken over the global development, regulatory approvals, manufacturing, and commercialization of Repotrectinib.
In addition to its work in the U.S. and other global markets, BMS collaborates with Zai Lab, a biopharmaceutical company based in China. Through an exclusive licensing agreement, Zai Lab is responsible for developing and marketing Repotrectinib in Greater China—including mainland China, Hong Kong, Taiwan, and Macau. This partnership has led to the successful approval of Repotrectinib in China, expanding access to patients in the region. Together, these companies are helping bring this promising targeted therapy to more people worldwide.
Learn more about Non-Small Cell Lung Cancer: Causes, Symptoms, Diagnosis, Treatment Options, and Latest 2025 Advances in Targeted and Immunotherapy on OncoDaily.
How does Repotrectinib work?
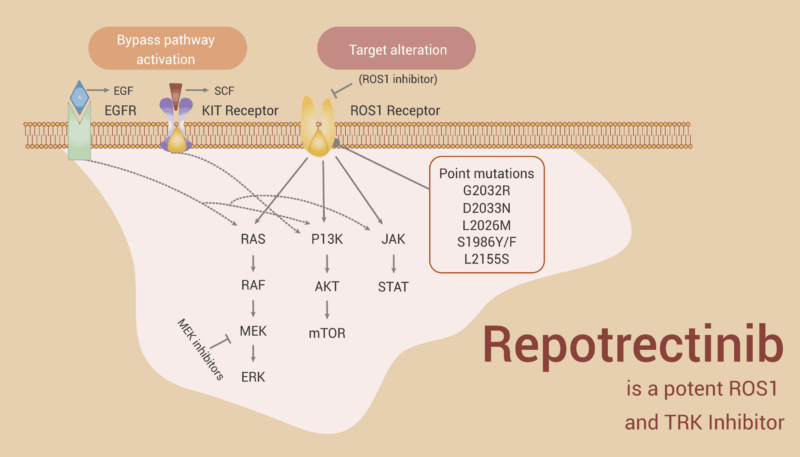
Repotrectinib is a next-generation targeted cancer therapy designed to block abnormal proteins that help cancer cells grow. Specifically, it works by inhibiting ROS1 and NTRK gene fusions—genetic changes found in some types of cancer that lead to the continuous activation of proteins called tyrosine kinases. These overactive proteins act like stuck gas pedals, making cancer cells grow and divide uncontrollably.
Augtyro is classified as a tyrosine kinase inhibitor (TKI). It binds tightly to the altered proteins caused by ROS1 or NTRK gene fusions, turning off their cancer-promoting signals. What makes Repotrectinib unique is that it was specially designed to be small and compact, which helps it overcome certain types of drug resistance and allows better penetration into the brain—a common site of cancer spread in ROS1-positive lung cancer.
By targeting these specific genetic drivers, Repotrectinib helps slow down or stop tumor growth, and in many cases, shrink tumors altogether. It offers a more precise, personalized approach to cancer treatment, especially for patients whose cancers have these rare gene alterations.
source: Network of Cancer Research
What Cancers Is Repotrectinib Approved to Treat?
Repotrectinib (Augtyro) is FDA-approved to treat cancers driven by specific genetic changes. In November 2023, it was approved for ROS1-positive non-small cell lung cancer (NSCLC) that is locally advanced or metastatic. This includes both newly diagnosed patients and those who previously received ROS1-targeted therapies. It showed strong results, including in patients with brain metastases.
In June 2024, Repotrectinib received accelerated approval for solid tumors with NTRK gene fusions in patients aged 12 and older. This includes any advanced cancer where standard treatments no longer work, regardless of where the tumor started. These approvals highlight Repotrectinib as a personalized treatment option for patients with rare but targetable mutations.

Learn more about Pancreatic Cancer: Symptoms, Causes, Types, Diagnosis & Treatment on OncoDaily.
What research is behind the approval?
Before a cancer treatment is approved, it must be studied carefully to show that it can safely help patients and improve their outcomes. Augtyro was tested in people whose tumors have specific genetic changes, and these studies formed the basis for its approvals.
Repotrectinib for Lung Cancer
Augtyro was approved by the FDA in November 2023 for locally advanced or metastatic ROS1-positive non-small cell lung cancer (NSCLC), based on the TRIDENT-1 trial. This global study included both patients new to ROS1-targeted therapy and those previously treated with a ROS1 TKI.
In TKI-naïve patients, the response rate was 79%, with a median response lasting over 34 months. In those previously treated, the response rate was 38%, lasting nearly 15 months. Repotrectinib also worked in patients with brain metastases and tumors with resistance mutations like ROS1 G2032R.
In 2024, updated TRIDENT-1 results were published in The New England Journal of Medicine, confirming long-term benefit: nearly 36 months of progression-free survival in TKI-naïve patients and 9 months in pretreated patients. Common side effects were mild to moderate and included dizziness, taste changes, nerve symptoms, constipation, and fatigue. Few patients stopped treatment due to side effects. The recommended dose is 160 mg once daily for 2 weeks, then increased to 160 mg twice daily until progression or side effects become unacceptable.

Repotrectinib for NTRK fusion–positive solid tumors
The FDA granted accelerated approval to Augtyro in June 2024 for patients aged 12 and older with advanced NTRK fusion–positive solid tumors who have either progressed after prior treatment or lack other options.
This approval was based on the TRIDENT-1 trial, which evaluated 88 adults with NTRK-driven cancers. Among those who had never received a TRK inhibitor, 58% responded to treatment, and most responses were still ongoing at the time of analysis. In patients previously treated with TRK inhibitors, the response rate was 50%, with responses lasting a median of 9.9 months.
These results highlight repotrectinib’s potential to provide meaningful and lasting benefit in both treatment-naïve and pretreated patients with NTRK fusion–positive cancers.
You can read full information about approval on FDA Official Website.
Repotrectinib side effects and its management
Like all cancer treatments, Repotrectinib may cause side effects. These effects vary from person to person and can depend on your overall health, other medications you’re taking, and whether you’ve previously received similar therapies. Many patients tolerate Repotrectinib well, but it’s important to be aware of the possible side effects, how to recognize them early, and how they can be managed to keep treatment on track.
Common Side Effects
The most frequently reported side effects of Augtyro include dizziness, changes in taste (also known as dysgeusia), constipation, fatigue, and shortness of breath. Some patients may also experience muscle pain, nausea, or a mild decrease in appetite. Dizziness is particularly common and can sometimes affect daily activities, especially early in treatment. Most of these side effects are mild to moderate and tend to become more manageable over time or with supportive care.
Less Common Side Effects
Less common but more serious side effects include liver enzyme elevations, low levels of white blood cells (which can increase infection risk), anemia, and vision changes such as blurred vision. Rarely, Repotrectinib can cause inflammation in the lungs known as pneumonitis or affect heart rhythm, leading to QT prolongation. While these events are uncommon, they may require closer monitoring or, in some cases, temporary interruption or dose adjustment of the medication.
Managing Side Effects
Managing side effects starts with open communication between patients and healthcare teams. Dizziness can often be reduced by rising slowly from sitting or lying positions and avoiding sudden movements. Taste changes may improve with dietary adjustments, such as using lemon juice or herbs to enhance flavor. Constipation can be managed through hydration, fiber intake, and, if needed, stool softeners or laxatives.
For fatigue, light physical activity and rest balance may help, while anti-nausea medications can be prescribed for ongoing nausea. Liver function and blood counts should be monitored through regular blood tests, and any abnormalities may prompt changes in dosage or temporary pauses in treatment. Vision changes or new respiratory symptoms should be reported right away, as they may require urgent evaluation.
In most cases, side effects can be controlled or reversed, allowing patients to continue treatment safely. Your care team will work closely with you to adjust your care plan as needed and ensure you feel supported throughout your therapy with Augtyro.

What is the Recommended Dosage of Repotrectinib?
Repotrectinib treatment typically begins with a dose of 160 mg once daily for the first 14 days. If well tolerated, the dose is then increased to 160 mg twice daily, which becomes the ongoing maintenance dose. This schedule is used for both ROS1-positive non-small cell lung cancer and NTRK fusion–positive solid tumors.
If side effects become difficult to manage, the dose can be reduced step by step—first to 120 mg and then to 80 mg, either once or twice daily, depending on the phase of treatment. In some cases, like serious liver problems or lung inflammation, treatment may need to be paused or permanently stopped.
Learn more about Colorectal Cancer: Epidemiology, Pathogenesis, Diagnosis, and Therapeutic Advances on OncoDaily.
How is Repotrectinib administered?
Repotrectinib should be taken at the same time each day, with or without food, as part of a consistent daily routine. The capsules must be swallowed whole—never opened, chewed, crushed, or dissolved—as altering them can affect how the medication works. If a capsule appears broken or damaged, it should not be taken. If a dose is missed or vomited, it’s best to skip that dose and continue with the next one at the usual scheduled time rather than trying to make it up.
The medication should be stored at room temperature, ideally between 20°C and 25°C, though short-term exposure to slightly cooler or warmer conditions is acceptable. Keeping the medicine stored properly ensures its effectiveness and safety throughout treatment.
What to Avoid During Repotrectinib Treatment?
During Repotrectinib treatment, patients should avoid concomitant use of strong or moderate CYP3A4 inhibitors or inducers, as these can significantly alter the drug’s metabolism, potentially increasing toxicity or reducing therapeutic efficacy. Grapefruit and grapefruit juice should also be avoided due to their known interaction with CYP3A4 enzymes, which may affect drug levels.
Alcohol consumption is generally discouraged during therapy, given the increased risk of hepatotoxicity. Additionally, because Augtyro may cause central nervous system effects such as dizziness, patients should exercise caution when engaging in activities requiring full alertness, including driving or operating machinery.
Healthcare providers should carefully review all concomitant medications, supplements, and lifestyle factors with patients to minimize the risk of interactions and ensure safe administration throughout the course of treatment.
How Long Does the Drug Stay in the Body?
Repotrectinib is primarily metabolized by the liver enzyme CYP3A4, with some processing through glucuronidation. After a single 160 mg dose, it is cleared from the body at about 15.9 liters per hour, and its half-life is approximately 40 hours. Most of the drug (around 89%) is excreted in the feces—about half unchanged—while a small portion (about 5%) is eliminated in the urine, mostly as metabolites.

Learn more about Stomach Cancer: Symptoms and Causes, Types, Diagnosis and Treatment on OncoDaily.
Repotrectinib effectiveness over time
The TOTEM trial (NCT04772235), a phase I study investigating the combination of repotrectinib and osimertinib in patients with EGFR-mutant non-small cell lung cancer (NSCLC) resistant to prior tyrosine kinase inhibitors, provides important insights into the drug’s effectiveness over time. Presented at the 2025 ASCO Annual Meeting and published in the Journal of Clinical Oncology, early results from 15 patients showed that repotrectinib, when combined with osimertinib, achieved a median progression-free survival of 4.4 months.
Notably, the combination demonstrated durable intracranial responses, with complete brain metastasis remission in nearly 43% of affected patients. The overall objective response rate was 33%, and more than half of the patients maintained stable disease during the study period. These findings highlight repotrectinib’s sustained antitumor activity, particularly in challenging cases with brain involvement and diverse EGFR mutations. The ongoing trial continues to evaluate the long-term benefits and safety of repotrectinib as part of combination therapy in this resistant patient population.
Ongoing trials with Repotrectinib
The Phase II REPOSE trial (NCT06315010) is evaluating repotrectinib in patients with ROS1-positive NSCLC and active brain metastases. This single-arm study will enroll 20 patients who may have had prior non-ROS1 treatments. The primary aim is to measure intracranial response. Treatment continues until progression or withdrawal, with follow-up for safety and survival.
FAQ
What is Repotrectinib?
Repotrectinib is a targeted cancer therapy that blocks abnormal proteins driving tumor growth in ROS1-positive lung cancer and NTRK fusion–positive tumors.
What cancers does Augtyro treat?
Augtyro is approved for ROS1-positive non-small cell lung cancer (NSCLC) and NTRK fusion–positive solid tumors in patients aged 12 and older, including cancers that have spread or are resistant to prior treatments.
Are there serious side effects of Augtyro?
Serious but less common effects include liver problems, low white blood cells, anemia, blurred vision, lung inflammation, and heart rhythm changes.
How is Augtyro taken?
Repotrectinib is taken orally as capsules, starting at 160 mg once daily for 2 weeks, then 160 mg twice daily if tolerated. Capsules must be swallowed whole.
What should be avoided while taking Repotrectinib?
While taking Augtyro, avoid grapefruit, strong CYP3A4 drugs, and alcohol. Use caution with activities requiring full alertness, like driving.
What is the half-life of Repotrectinib?
Augtyro has a half-life of approximately 40 hours, meaning it takes about 40 hours for half of the drug to be cleared from the body.


