Elacestrant (brand name Orserdu) is a breakthrough oral medication for treating advanced or metastatic estrogen receptor-positive (ER+), HER2-negative breast cancer. Approved by the U.S. Food and Drug Administration (FDA) on January 27, 2023, Elacestrant is indicated for postmenopausal women and adult men with ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer whose disease has progressed after at least one line of endocrine therapy.
What Is Elacestrant and How Does It Work?
Elacestrant (Orserdu) is an advanced hormonal therapy designed to treat certain types of breast cancer. It belongs to a class of drugs called selective estrogen receptor degraders (SERDs). This means that instead of just blocking estrogen from fueling cancer growth, like some older treatments do, Elacestrant actually breaks down the estrogen receptor itself.
This is especially important for patients with ER-positive, HER2-negative breast cancer who have developed a specific mutation known as ESR1. This mutation makes cancer cells resistant to standard hormonal treatments, such as aromatase inhibitors and selective estrogen receptor modulators (SERMs). By directly reducing the number of estrogen receptors on cancer cells, Elacestrant helps slow down or stop their growth, even in cases where other treatments have stopped working.
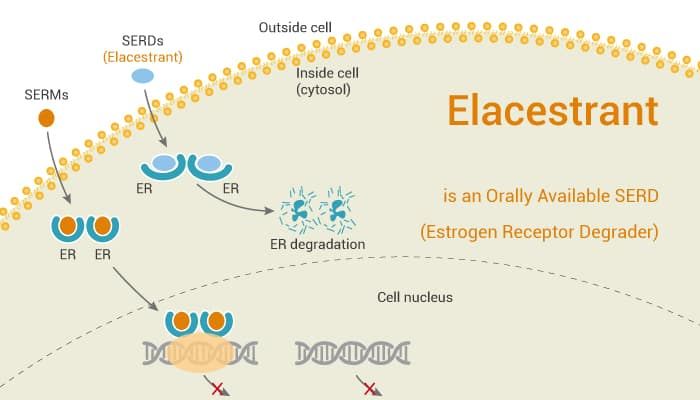
Photo from LinkedIn
What Is a Clinical Trial and Why Does It Matter?
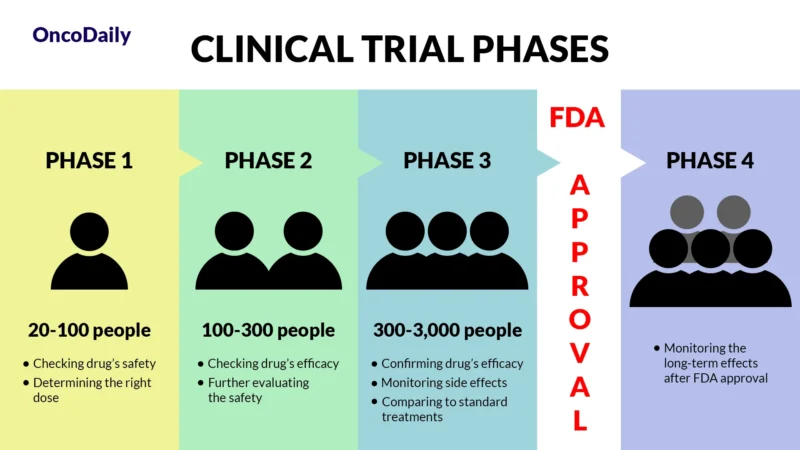
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Elacestrant was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.
What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
What Cancers Does Elacestrant Treat?
Elacestrant is FDA-approved to treat:
- Advanced or metastatic ER-positive, HER2-negative breast cancer
- Patients with ESR1 mutations who have experienced disease progression after at least one line of endocrine therapy.

You can read more about Breast Cancer: Symptoms Causes, Stages, Diagnosis and Treatment on OncoDaily.
Efficacy and Clinical Trial Results
The FDA approval of Elacestrant was based on the EMERALD trial, a phase III study assessing its efficacy in ER-positive, HER2-negative advanced breast cancer patients. The trial compared Elacestrant to standard-of-care endocrine therapy in patients pretreated with CDK4/6 inhibitors and chemotherapy.
Key Findings from the EMERALD Trial
- Progression-Free Survival (PFS): 30% reduction in the risk of disease progression (hazard ratio [HR] = 0.70), In ESR1-mutated patients, PFS improved significantly (HR = 0.55)
- Overall Survival (OS): Early data suggests a trend toward improved survival benefits
- Side Effects: Nausea (35%), fatigue (20%), and increased cholesterol levels, but most were manageable.
These results highlight Elacestrant as the first oral SERD to significantly prolong PFS in ESR1-mutated breast cancer patients.
You can read more about the standard of care for hormone receptor-positive, HER2-negative metastatic breast cancer on OncoDaily.
Ongoing Clinical Trials and Research
Elacestrant is being investigated in combination with other therapies to enhance treatment outcomes:
- ELECTRA Study (NCT05386108): Examining Elacestrant with Abemaciclib (CDK4/6 inhibitor) in metastatic breast cancer patients, including those with brain metastases.
- TREAT ctDNA Study (Phase III): Evaluating adjuvant Elacestrant versus standard endocrine therapy in ER+/HER2- breast cancer patients with circulating tumor DNA (ctDNA) relapse.
FDA Approval and Patient Access
The FDA approval of Elacestrant provides a new oral treatment option for patients with ESR1-mutated breast cancer, potentially delaying chemotherapy use. Its availability offers more flexibility and convenience compared to injectable SERDs like Fulvestrant.
Elacestrant Dosage & Administration Guide
Elacestrant is taken once daily by mouth with food to help prevent nausea. The tablet should be swallowed whole—do not chew, crush, or break it. Damaged tablets should not be used.
For best results, take Elacestrant at the same time each day. If you miss a dose by more than six hours, skip it and take your next scheduled dose. If vomiting occurs after taking a dose, do not take an extra pill—just continue with your regular schedule.
Elacestrant Dosage
The usual dose of Elacestrant is 345 mg once daily. If side effects occur, doctors may lower the dose to 258 mg, and if needed, further reduce it to 172 mg. If the lowest dose is not tolerated, treatment may be stopped. Elacestrant is available in 86 mg and 345 mg tablets, allowing for dose adjustments based on individual patient needs.
Side Effects and Management
Elacestrant, a selective estrogen receptor degrader (SERD) used for ER-positive metastatic breast cancer, has several side effects that require careful management. Here’s how to manage them:
Common Side Effects
- Nausea & Vomiting: Take with food and use anti-nausea medications if needed
- Fatigue & Muscle Pain: Stay hydrated and incorporate light exercise
- Diarrhea & Constipation: Adjust diet and consider medications if persistent
- Increased Cholesterol & Liver Enzymes: Regular blood tests to monitor levels
Serious Side Effects (Rare)
- QT Prolongation: Regular ECG monitoring is recommended
- Liver Toxicity: Routine liver function tests should be done

What to Expect Long-Term
Elacestrant helps manage cancer but is not a cure. Clinical trials suggest its benefits last several months longer than standard endocrine therapy, particularly in patients with ESR1 mutations. If the disease progresses, other targeted therapies or chemotherapy may be considered.
Drug Interactions and Precautions
When taking elacestrant, it’s important to be aware of potential drug interactions and necessary precautions. Certain medications, such as strong CYP3A4 inhibitors—including some antibiotics and antifungal drugs—as well as CYP3A4 inducers, can interfere with the drug’s metabolism, potentially altering its effectiveness.
For patients who are pregnant or breastfeeding, elacestrant poses a risk to the developing fetus, making effective contraception essential both during treatment and for at least one week after the last dose.
Additionally, handling the medication properly is crucial. Tablets should be taken whole—broken or crushed tablets should never be consumed, as this could affect drug absorption and safety.
Real-World Effectiveness and Future Prospects
Real-world data and ongoing studies indicate Elacestrant’s strong potential as a standard treatment for ESR1-mutated breast cancer. Future research may expand its use in early-stage breast cancer and combination therapies, offering more options for patients.
The Future of Breast Cancer Treatment with Elacestrant
With ongoing research, Elacestrant may be combined with other targeted therapies such as CDK4/6 inhibitors, PI3K inhibitors, and antibody-drug conjugates (ADCs) to further improve patient outcomes. Future trials may also explore its role in earlier-stage breast cancer and other hormone-driven cancers.
In conclusion, Elacestrant is a promising new treatment for ER-positive, HER2-negative, ESR1-mutated breast cancer. Its oral administration, efficacy in delaying disease progression, and manageable side effects make it a valuable option for patients seeking alternatives to injectable endocrine therapies. Ongoing research continues to explore its full potential, offering hope for improved cancer care in the future.

You can read about Amy Dowden and Breast Cancer: How She Went Against It, How She Survived on OncoDaily.
What Other SERDs Are Available and How They Work
SERDs are a class of drugs designed to treat estrogen receptor-positive (ER+) breast cancer by targeting and degrading the estrogen receptor (ER), a key driver of tumor growth. Here’s a step-by-step breakdown of their mechanism. SERDs bind to the ER with high affinity, competing with estrogen (estradiol) for the receptor’s ligand-binding domain. Unlike selective estrogen receptor modulators (SERMs, e.g., tamoxifen), which block estrogen activity but may partially activate ER in some tissues, SERDs act as pure antagonists.
Upon binding, SERDs induce structural changes in the ER. These changes destabilize the receptor, preventing it from adopting an active conformation required for dimerization (pairing of two ER molecules) and interaction with coactivator proteins. The destabilized SERD-ER complex recruits cellular machinery, such as ubiquitin ligases, which tag the receptor for degradation via the proteasome (the cell’s protein-recycling system). This process reduces ER levels in cancer cells, effectively shutting down estrogen signaling.
By degrading ER, SERDs eliminate a critical pathway for cancer cell proliferation and survival. This is particularly effective in tumors resistant to other endocrine therapies (e.g., aromatase inhibitors or SERMs), where ER mutations or ligand-independent activation pathways (e.g., PI3K-AKT-mTOR signaling) allow tumors to bypass traditional treatments. SERDs offer several advantages over other therapies. They address resistance mechanisms like ER mutations or alternative signaling pathways by removing the receptor entirely, unlike SERMs or AIs, which only block estrogen or reduce its production. Additionally, SERDs not only antagonize ER but also induce its degradation, offering a more comprehensive suppression of ER-driven cancer growth.
Fulvestrant, the first FDA-approved SERD (2007), is administered via intramuscular injection and is used for advanced/metastatic ER+ breast cancer. Next-generation oral SERDs, such as elacestrant and camizestrant, are being developed to improve oral bioavailability and efficacy. These drugs use innovative designs, such as PROTAC (proteolysis-targeting chimera) technology, which links ER-binding molecules to degrons (degradation-inducing components) for enhanced receptor targeting. Research focuses on expanding SERDs’ utility through the development of oral formulations to replace injections, combination therapies with CDK4/6 inhibitors or PI3K inhibitors to enhance antitumor effects, and novel scaffolds to improve pharmacokinetics and reduce side effects.
By degrading ER and halting its cancer-promoting signals, SERDs represent a critical tool in managing ER+ breast cancer, especially in resistant or recurrent cases.
If you’re a healthcare provider, access the professional version here.
Written by Mariam Khachatryan, MD


