Elacestrant is an oral selective estrogen receptor degrader (SERD) used to treat advanced or metastatic estrogen receptor-positive (ER+), HER2-negative breast cancer. Elacestrant was approved by the FDA on January 27, 2023, for the treatment of postmenopausal women and adult men with ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer who have experienced disease progression following at least one line of endocrine therapy, particularly those with ESR1 mutations.
Which company produced Elacestrant?
Elacestrant was originally discovered by Eisai Co., Ltd. Eisai granted an exclusive worldwide license for elacestrant to Radius Health, Inc., which later transferred the global licensing rights to the Menarini Group in July 2020. The Menarini Group, through its subsidiary Stemline Therapeutics, is now responsible for the global registration, commercialization, and further development of elacestrant, marketed under the brand name ORSERDU
How does Elacestrant work?
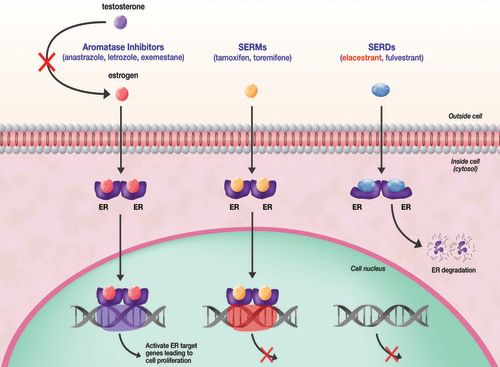
Elacestrant (Orserdu) is a selective estrogen receptor degrader (SERD) that works by targeting and degrading estrogen receptor-alpha (ERα), which is critical for the growth of ER-positive, HER2-negative breast cancer cells. Unlike traditional endocrine therapies that simply block estrogen signaling, elacestrant destabilizes and promotes the degradation of the estrogen receptor, reducing its presence on cancer cells. This leads to a decrease in estrogen-driven cancer cell proliferation, particularly in patients with ESR1 mutations, which are associated with resistance to standard endocrine therapies such as aromatase inhibitors and selective estrogen receptor modulators (SERMs).
Bardia, A., Aftimos, P., Bihani, T., Anderson-Villaluz, A. T., Jung, J., Conlan, M. G., & Kaklamani, V. G. (2019). EMERALD: Phase III Trial of Elacestrant (RAD1901) vs Endocrine Therapy for Previously Treated ER+ Advanced Breast Cancer. Future Oncology, 15(28), 3209–3218. https://doi.org/10.2217/fon-2019-0370
What Cancers Is Elacestrant Approved to Treat?
Elacestrant (Orserdu) is FDA-approved for the treatment of advanced or metastatic estrogen receptor-positive (ER+), HER2-negative breast cancer in postmenopausal women and adult men. It is specifically indicated for patients with ESR1 mutations whose disease has progressed after at least one line of endocrine therapy.

Read more about Breast Cancer: Symptoms, Causes, Stages, Diagnosis and Treatment on OncoDaily
What research is behind the approval?
The EMERALD Trial, published in JCO on May 18, 2022, evaluated the efficacy of Elacestrant, a novel oral selective estrogen receptor degrader, in treating ER-positive, HER2-negative advanced breast cancer in patients who had been treated with one or two lines of endocrine therapy.
This phase III trial included patients pretreated with a cyclin-dependent kinase 4/6 inhibitor and, in some cases, chemotherapy. Patients were randomly assigned to receive either elacestrant (400 mg daily) or standard-of-care (SOC) endocrine therapy. The primary endpoint was progression-free survival (PFS), assessed in all patients and those with ESR1 mutations.
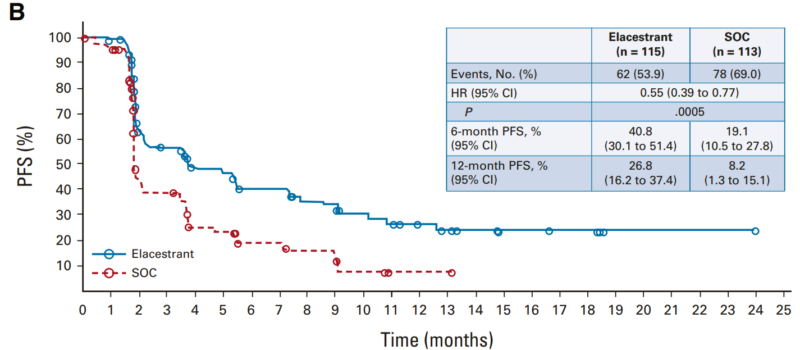
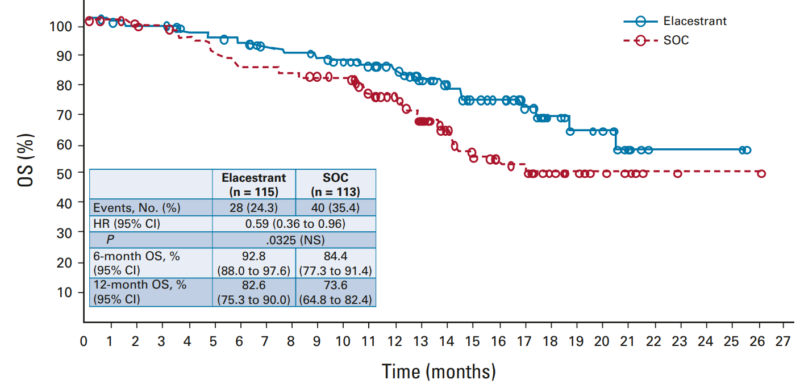
PFS and OS in the group of ESR1 mutations
The results showed that elacestrant significantly prolonged PFS, with a hazard ratio of 0.70 in the overall group and 0.55 in patients with ESR1 mutations. Although Elacestrant was linked to more treatment-related adverse events, including nausea (35%) and grade 3/4 events (7.2%), these were manageable. Treatment discontinuation due to adverse events occurred in 3.4% of elacestrant-treated patients versus 0.9% in the SOC group.
Elacestrant is the first oral selective estrogen receptor degrader to significantly improve PFS, particularly in ESR1-mutated patients, leading to its FDA approval for ER-positive, HER2-negative advanced breast cancer.
Elacestrant Combinations and Treatment Outcomes
Elacestrant is being explored in combination with other therapies to improve treatment outcomes for ER-positive, HER2-negative advanced breast cancer, particularly in patients with ESR1 mutations.
The ELECTRA study (NCT05386108) evaluates the combination of Elacestrant and Abemaciclib in patients with ER+, HER2- metastatic breast cancer (mBC), focusing on brain metastases. Published in the Journal of Clinical Oncology on May 29, 2024, phase 1b results showed no dose-limiting toxicities, with the recommended phase 2 dose (RP2D) to be reported. The most common side effects included diarrhea (81%), nausea (62%), and neutropenia (50%).
Preliminary findings revealed partial responses in 6 out of 21 evaluable patients, with 9 showing stable disease. Phase 2 will assess the combination’s efficacy and safety, particularly in patients with brain metastases, as both drugs cross the blood-brain barrier. Early data suggest promising antitumor activity and manageable safety, positioning Elacestrant as a potential backbone for combination therapies.

Read more about Immunotherapy for Breast Cancer: Types, Success Rate, Side Effects on OncoDaily.
Elacestrant side effects and its management
Elacestrant, a selective estrogen receptor degrader (SERD) used for ER-positive metastatic breast cancer, has several side effects that require careful management. The most common issues include nausea, vomiting, and diarrhea, which can be alleviated with antiemetics, dietary adjustments, and hydration. Constipation and abdominal discomfort may be managed with fiber intake, hydration, and mild laxatives if needed.
Fatigue and muscle or joint pain are also frequent, with rest, light exercise, and pain relievers like NSAIDs or acetaminophen helping to reduce discomfort. Headaches can be addressed with hydration and over-the-counter pain relievers. Additionally, Elacestrant can cause laboratory abnormalities, such as elevated cholesterol, liver enzymes, and anemia, necessitating routine blood monitoring and, if needed, dietary modifications or medications.
Rare but serious effects include QT prolongation and liver toxicity, requiring close monitoring and, in severe cases, treatment adjustments. By staying vigilant and addressing side effects early, patients can better tolerate treatment while maintaining their quality of life.

What is the Recommended Dosage of Elacestrant
The recommended dose of Elacestrant is 345 mg once daily, continued until disease progression or unacceptable toxicity.
For adverse reactions, the dose can be reduced to 258 mg daily, then 172 mg daily if needed. If a patient cannot tolerate 172 mg/day, treatment should be discontinued. Elacestrant is available in 86 mg and 345 mg tablets for flexible dosing.
How is Elacestrant administered?
Elacestrant is taken orally once daily with food to minimize nausea and vomiting. It should be swallowed whole at the same time each day without chewing, crushing, or splitting. Damaged tablets should not be used. If a dose is missed by more than 6 hours or vomiting occurs, skip it and take the next dose as scheduled. Store at 20-25°C (68-77°F), with short-term exposure to 15-30°C (59-86°F) allowed.
What to Avoid During Elacestrant Treatment?
During Elacestrant treatment, avoid certain substances to ensure its effectiveness. CYP3A4 metabolizes Elacestrant, so avoid strong or moderate CYP3A4 inhibitors (like some antifungals or antibiotics) and inducers, as they can affect drug levels.
Elacestrant can harm a fetus, so women of reproductive potential should use contraception during treatment and for a week after, as should men with pregnant partners. Breastfeeding is also not recommended during treatment and for one week after.
Do not take broken or damaged tablets, as this could affect the drug’s effectiveness. Always consult your healthcare provider before starting new medications or supplements to avoid interactions.
Elacestrant effectiveness over time
The EMERALD trial, published in Clinical Cancer Research in 2024, showed that Elacestrant significantly improved progression-free survival (PFS) compared to standard-of-care (SOC) in ER-positive, HER2-negative metastatic breast cancer with ESR1 mutations. In patients who had prior treatment with endocrine therapy and CDK4/6 inhibitors for at least 12 months, Elacestrant extended PFS to 8.6 months versus 1.9 months with SOC. These results highlight Elacestrant as a promising treatment option.
A retrospective study analyzed the impact of CDK4/6 inhibitors (CDK4/6i) and novel agents on patients (pts) with stage IV de novo HR+ve/HER2-ve breast cancer (BC). Using data from the TriNetX Research Network, 7,025 pts were identified. The median overall survival (OS) was 6.8 years, with Ribo and Abema showing better OS compared to Palbo. Patients receiving ELA had significantly improved OS (HR: 0.30; p=0.0001). Additionally, ELA + fulvestrant had better outcomes than fulvestrant alone (p=0.0022). Oral SERDs and ADCs were associated with improved prognosis. These findings were presented at the ESMO Congress 2024.
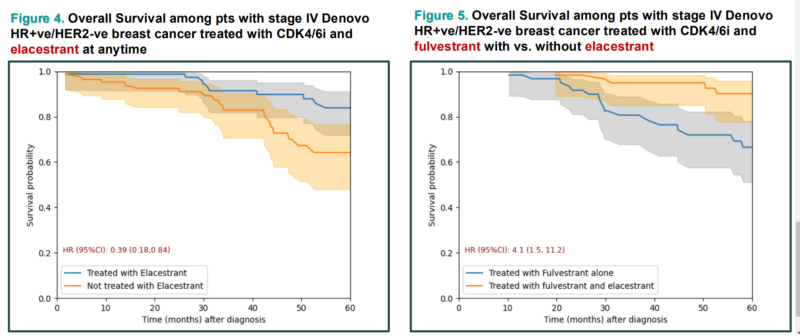
Ongoing trials with Elacestrant
The ELEMENT trial is a phase II study assessing whether adding elacestrant (SERD) to olaparib (PARP inhibitor) improves outcomes in HR+/HER2- metastatic breast cancer with gBRCA1/2 mutations. Patients are randomized 2:1 to receive olaparib with or without elacestrant until disease progression or toxicity. By combining endocrine and DNA repair targeting, the trial aims to enhance progression-free survival in this high-risk group. Regular monitoring includes blood tests, imaging, and quality-of-life assessments.
The TREAT ctDNA study is a phase III trial evaluating adjuvant Elacestrant vs standard endocrine therapy (ET) in ER+/HER2- breast cancer patients with ctDNA relapse. This multicenter, randomized trial screens 1,960 high-risk patients for ctDNA relapse, followed by the randomization of 220 ctDNA-positive patients to continue ET or receive Elacestrant for 2 years. The primary endpoint is distant metastasis-free survival, with secondary endpoints including invasive disease-free survival and overall survival. Recruitment began in December 2023, with expansion to 11 countries in 2024. Presented at ESMO Congress 2024.
Written by Mariam Khachatryan, MD
FAQ
What is Elacestrant used for?
Elacestrant is used to treat ER-positive, HER2-negative metastatic breast cancer in patients with ESR1 mutations who have progressed on prior endocrine therapy.
How does Elacestrant work?
Elacestrant is a selective estrogen receptor degrader (SERD) that binds to estrogen receptors, leading to their degradation and reducing cancer cell growth.
What clinical trial led to Elacestrant’s approval?
Elacestrant was approved based on the EMERALD trial, which showed improved progression-free survival compared to standard endocrine therapy in ESR1-mutated breast cancer.
What are the most common side effects of Elacestrant?
Common side effects include nausea, fatigue, hot flashes, joint pain, decreased appetite, and vomiting.
What should I avoid while taking Elacestrant?
Avoid grapefruit and grapefruit juice, as they can affect how elacestrant is metabolized. Also, discuss any other medications or supplements with your doctor.
What is the recommended dosage of Elacestrant?
The standard dose is 345 mg taken orally once daily with food, but your doctor may adjust it based on tolerance and side effects.
Is elacestrant better than fulvestrant?
Elacestrant offers the advantage of oral administration and has shown improved efficacy in ESR1-mutated breast cancer compared to fulvestrant in the EMERALD trial.


