Binimetinib is an oral targeted cancer therapy that belongs to a class of drugs known as MEK inhibitors. It works by blocking MEK1 and MEK2, proteins part of the MAPK/ERK signaling pathway, often overactive in specific cancers. By interfering with this pathway, binimetinib can slow or stop the growth of cancer cells. Binimetinib is primarily used to treat unresectable or metastatic melanoma.
Which company produced Binimetinib?
Binimetinib (brand name Mektovi) was developed by Array BioPharma, a biopharmaceutical company based in Boulder, Colorado, USA. Array BioPharma specializes in discovering, developing, and commercializing targeted small-molecule drugs for treating cancer and other serious diseases. The company focused heavily on therapies that targeted specific genetic mutations and pathways involved in cancer progression.
In 2010, Array entered into a licensing agreement with Novartis, granting the Swiss pharmaceutical company exclusive worldwide rights to develop and commercialize binimetinib. In December 2014, Array BioPharma reached an agreement to regain full rights to binimetinib from Novartis, following an FTC directive tied to Novartis’ acquisition of GSK’s oncology assets. The deal, finalized in March 2015, included an $85 million payment to Array and transitional support from Novartis.
In 2019, Array BioPharma was acquired by Pfizer Inc., one of the world’s largest and most well-known pharmaceutical companies. With this acquisition, Pfizer expanded its oncology pipeline and gained access to key targeted therapies like Binimetinib (a MEK inhibitor) and Encorafenib (a BRAF inhibitor), enhancing its capabilities in precision medicine for cancer care.
How does Binimetinib work?
Binimetinib works by quietly stepping into the cancer cell’s internal communication system and cutting off a critical signal. Specifically, it targets two key proteins—MEK1 and MEK2—that normally act as messengers, passing along growth instructions to another protein called ERK. In many cancers, especially those with BRAF mutations, this signaling chain becomes hyperactive, sending constant commands for the cell to grow and divide uncontrollably.
By selectively inhibiting MEK1 and MEK2, Mektovi prevents them from activating ERK, effectively silencing the downstream signal that drives tumor growth. As a result, cancer cells slow down their rapid division, some enter programmed cell death (apoptosis), and overall, the tumor may stop growing—or even begin to shrink.
What makes binimetinib especially powerful is when it’s used in combination with encorafenib, a drug that targets the faulty BRAF protein higher up in the pathway. While encorafenib shuts off the source of the problem, binimetinib ensures that any remaining signals don’t sneak through. Together, they create a complete blockade of the pathway, helping to delay resistance and deliver a longer-lasting response for patients with BRAF-mutant cancers.

Fernandez, Manuel & Choi, Jacob & Sosman, Jeffrey. (2023). New Approaches to Targeted Therapy in Melanoma. Cancers. 15. 3224. 10.3390/cancers15123224.
The MAPK Pathway
Mektovi targets the MAPK/ERK signaling pathway—a key cellular communication system that regulates essential processes such as cell growth, proliferation, differentiation, and survival. This pathway involves a series of proteins that pass signals from the cell surface to the DNA in the nucleus. The main components include:
- RAS – a protein that activates the pathway when a growth signal is received.
- RAF – activated by RAS, it phosphorylates MEK.
- MEK1/2 – proteins phosphorylated by RAF that in turn activate ERK.
- ERK – once activated, it enters the nucleus and promotes cell division and survival.
In many cancers, mutations—especially in BRAF (like the V600E mutation)—lead to constant activation of this pathway, causing uncontrolled cell division and tumor growth, even in the absence of growth signals.
What Cancers is Binimetinib approved to treat?
Mektovi is FDA-approved in combination with encorafenib for two indications. In 2018, it was approved for unresectable or metastatic melanoma with BRAF V600E or V600K mutations. In 2023, it received approval for metastatic non-small cell lung cancer (NSCLC) with a BRAF V600E mutation. These approvals highlight its role as an MEK inhibitor targeting the MAPK pathway in BRAF-mutated cancers.
What research is behind the approval?
In a pivotal Phase III trial published in the Journal of Clinical Oncology in 2016, binimetinib demonstrated clinical benefit for patients with NRAS-mutant metastatic melanoma—a subset with limited treatment options, especially after the failure of immunotherapy. The NEMO study (NCT01763164) compared binimetinib to dacarbazine in 402 patients and showed that binimetinib significantly improved progression-free survival, overall response rate (15% vs 7%), and disease control rate (58% vs 25%). These results positioned binimetinib as a potential option for NRAS-mutant melanoma, though it is not FDA-approved for this indication.
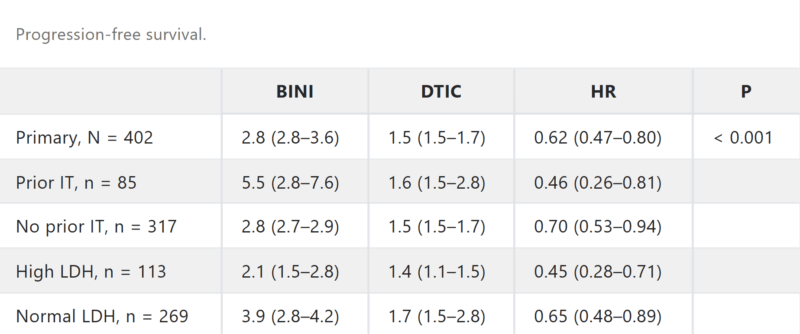
In a Phase 3 study (COLUMBUS) published in The Lancet Oncology in 2018, the combination of encorafenib (a BRAF inhibitor) and binimetinib (a MEK inhibitor) was compared to vemurafenib in patients with BRAFV600-mutant advanced melanoma. The study found that the combination therapy significantly improved progression-free survival (PFS), with a median PFS of 14.9 months for encorafenib + binimetinib versus 7.3 months for vemurafenib (HR 0.54, p<0.0001). Additionally, the combination showed a better tolerability profile compared to the other two treatments. These findings suggest that encorafenib + binimetinib could be a new treatment option for patients with BRAF-mutant melanoma.
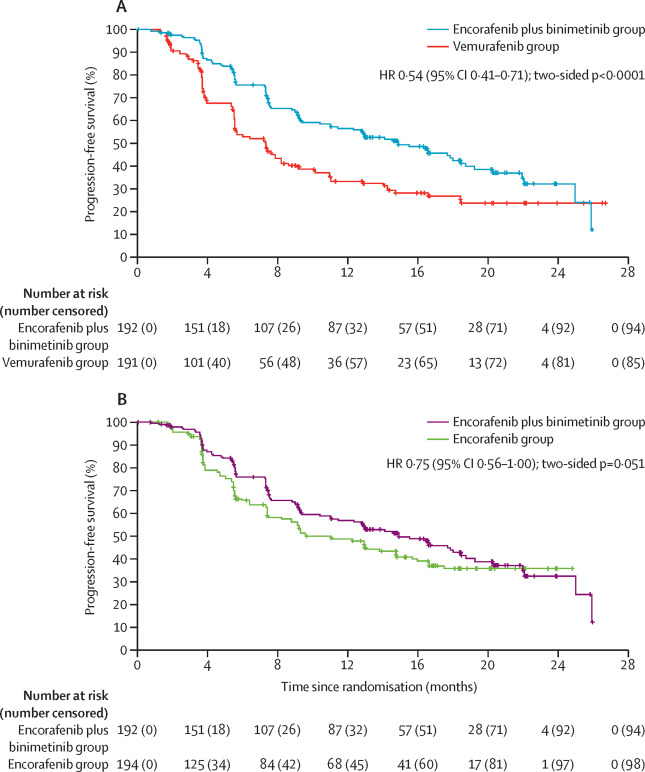
The Phase II PHAROS study (NCT03915951), published in JCO in 2023, assessed encorafenib plus binimetinib in BRAFV600E-mutant metastatic non-small-cell lung cancer (NSCLC). In 98 patients (59 treatment-naïve, 39 previously treated), the combination showed a 75% objective response rate (ORR) in treatment-naïve and 46% in previously treated patients. The disease control rate (DCR) at 24 weeks was 64% and 41%, respectively. Median progression-free survival (PFS) was not estimable for treatment-naïve patients but 9.3 months for previously treated ones. Common side effects included nausea, diarrhea, and fatigue. Overall, the combination demonstrated meaningful clinical benefits with a safety profile similar to melanoma treatment.
Binimetinib side effects and its management
Mektovi when used in combination with encorafenib, is an effective treatment for BRAF-mutant metastatic melanoma. However, it can cause a range of side effects, from common and manageable symptoms to rare but serious complications.
Common side effects
Among the most frequently reported side effects are fatigue, gastrointestinal issues such as nausea, vomiting, diarrhea, and abdominal discomfort, and muscle-related symptoms like muscle pain and elevated creatine kinase levels. Visual disturbances, including blurred vision, are also commonly observed, along with skin reactions like rashes and dry skin. Patients may also experience changes in blood counts and liver enzyme levels.
Less common side effects
Less commonly, binimetinib may lead to cardiovascular issues such as high blood pressure and heart-related complications. Some patients report ocular problems including retinal changes and other visual impairments. Respiratory symptoms like shortness of breath and coughing may also occur. Neurological effects such as dizziness and headaches are possible, as well as metabolic imbalances involving blood sugar and electrolytes.
Rare but Serious Side Effects
Though rare, there are serious risks associated with binimetinib use. These include interstitial lung disease, a potentially life-threatening lung condition, and retinal vein occlusion, a serious eye disorder that can affect vision. Some patients have developed rhabdomyolysis, a severe muscle breakdown that can lead to kidney damage, as well as inflammation of the pancreas (pancreatitis) and peripheral neuropathy, which causes weakness or numbness in the limbs.
Management of side effects
To manage these risks, regular monitoring is recommended. Patients typically undergo routine assessments of cardiac function to watch for early signs of cardiomyopathy. Eye exams are conducted to monitor for retinal issues, and liver function tests are frequently performed to ensure the liver is processing the drug safely. Muscle enzyme levels may also be checked to catch early signs of muscle damage. Patients must communicate any new or worsening symptoms to their healthcare provider right away.
Pregnancy and Breastfeeding
This drug may harm an unborn baby. Pregnancy should be avoided while on treatment and for 30 days afterward. Women should not breastfeed during treatment and for three days after the last dose.

What is the Recommended Dosage of Binimetinib?
Binimetinib is an oral MEK inhibitor available in 15 mg tablets. The recommended dose of Mektovi is 45 mg taken twice daily by mouth, in combination with Encorafenib, continued until disease progression or unacceptable toxicity. If encorafenib is permanently discontinued, binimetinib should also be stopped.
In cases where dose reduction is necessary due to side effects, the first step is to reduce the dose to 30 mg twice daily. If this reduced dose is not tolerated, treatment should be discontinued altogether.
Dose modifications are required for specific toxicities, including cardiac dysfunction, liver enzyme elevations, muscle-related side effects, eye disorders, and lung toxicity. In general, the drug may be withheld temporarily to allow recovery, then resumed at a reduced dose if the patient improves. In cases of severe or recurrent toxicity, Binimetinib should be permanently discontinued.
Learn more about Melanoma (Skin Cancer): Symptoms, Causes, Stages, Diagnosis and Treatment on OncoDaily.
How is Binimetinib Administered?
Binimetinib is taken orally twice daily, about 12 hours apart, with or without food. If a dose is missed, and it’s less than 6 hours before the next one, skip it—do not double up. If vomiting occurs after taking a dose, do not retake it; simply continue with the next scheduled dose.
Keep tablets at room temperature (20–25°C or 68–77°F), allowing short-term exposure between 15–30°C (59–86°F).
What to Avoid During Binimetinib Treatment?
During treatment with binimetinib, certain precautions should be taken to ensure safety and effectiveness. Patients should avoid becoming pregnant, as the drug may cause harm to an unborn baby. Effective birth control should be used during treatment and for at least 30 days after the last dose. Breastfeeding is also not recommended during treatment and for at least 3 days afterward, as it is unknown if the medication passes into breast milk.
Patients are advised to limit sun exposure while on binimetinib, as it can increase sensitivity to sunlight. Protective clothing and broad-spectrum sunscreen are recommended when spending time outdoors. Additionally, grapefruit and grapefruit juice should be avoided, as they may interfere with how the drug is processed in the body, potentially increasing side effects.
It’s important not to start or stop any medications, including over-the-counter drugs or supplements, without consulting a healthcare provider. Some medications, especially those that affect liver enzymes or heart rhythm, can interact with binimetinib and impact its safety or effectiveness.

Read more about Immunotherapy for Melanoma: Types, Success Rate, Side Effects on OncoDaily.
Binimetinib effectiveness over time
In the 7-year analysis of the COLUMBUS Phase III trial, published in the Journal of Clinical Oncology in 2024, encorafenib plus binimetinib showed significant long-term benefits for patients with BRAF V600E/K-mutant metastatic melanoma. After 7 years, progression-free survival (PFS) was 21.2% and overall survival (OS) was 27.4% in the combination therapy group, compared to 6.4% PFS and 18.2% OS in the vemurafenib group. The study also identified 34 long-term responders. These results underscore the long-term efficacy and safety of the encorafenib + binimetinib combination, solidifying its role in treating BRAF-mutant melanoma.
Ongoing trials with Binimetinib
ENCEFALO (NCT06887088) is a Phase II, single-arm, multicenter trial evaluating a two-step treatment for patients with BRAF-mutant melanoma and symptomatic brain metastases.
Patients receive encorafenib plus binimetinib for 8 weeks, followed by cemiplimab and fianlimab. The main goal is to assess the 6-month intracranial progression-free survival (icPFS) rate, using RECIST criteria. The trial aims to improve outcomes compared to historical data, targeting a 40% 6-month icPFS—double that seen in the CM204 trial’s symptomatic arm (20%).
CEBBRA (NCT06207656) is a Phase II trial testing cetuximab, encorafenib, and binimetinib as induction therapy in BRAF V600E-mutated MSS advanced colorectal cancer that is initially or potentially resectable. The study aims to improve outcomes in this high-risk group, building on prior data showing this combination outperforms standard treatments in later-line settings.
PERFUME (NCT06159478) is a Phase II, open-label trial evaluating binimetinib in patients with BRAF fusion–positive low-grade glioma or pancreatic cancer. The study includes two cohorts: recurrent low-grade glioma and advanced or recurrent pancreatic cancer. Patients receive oral binimetinib 45 mg twice daily to assess its safety and efficacy in these rare, hard-to-treat cancers.

Learn more about Brachytherapy for Skin Cancer: Types, Success Rate, Side Effects on OncoDaily.
Written by Mariam Khachatryan, MD
FAQ
What is Mektovi used for in cancer treatment?
Mektovi is used to treat BRAF-mutant metastatic melanoma and non-small cell lung cancer (NSCLC), usually in combination with encorafenib.
Is Binimetinib a targeted therapy?
Yes, Binimetinib is a targeted MEK inhibitor. It blocks the MEK1 and MEK2 proteins involved in the growth of cancer cells with BRAF mutations.
How does Binimetinib work?
Binimetinib targets the MAPK/ERK pathway, stopping signals that tell cancer cells to grow. This helps slow tumor growth and may shrink tumors.
What are the most common side effects of Binimetinib?
Common side effects include fatigue, nausea, diarrhea, rash, muscle pain, and vision changes. Most are manageable with monitoring and support.
What is the recommended dosage of Mektovi?
The usual dose is 45 mg taken orally twice a day, 12 hours apart, along with encorafenib for best results.
Can Binimetinib be taken with or without food?
Yes, Binimetinib can be taken with or without food. Just make sure to take it consistently every 12 hours as prescribed.
What should you do if you miss a dose of Binimetinib?
If you miss a dose and it's less than 6 hours to your next one, skip it. Don’t double the dose to make up for a missed pill.
Are there ongoing clinical trials involving Binimetinib?
Yes, current trials are exploring Binimetinib in brain metastases, colorectal cancer, pancreatic cancer, and low-grade gliomas with BRAF fusions.


