Anbenitamab (KN026) is a new targeted cancer medicine being developed for cancers that have HER2, a protein that helps cancer cells grow. Anbenitamab is not chemotherapy. It is a targeted antibody treatment designed to block HER2 more completely than older medicines.
Doctors are studying Anbenitamab (KN026) mainly for HER2-positive gastric (stomach) cancer and gastroesophageal junction (GEJ) cancer, and also for HER2-positive breast cancer. These cancers can become resistant to earlier HER2 treatments, such as trastuzumab. Anbenitamab was created to help patients whose cancer has stopped responding to standard HER2 therapy.
What Does “HER2-Positive” Mean?
HER2 is a protein found on the surface of some cancer cells. When there is too much HER2, the cancer can grow and spread faster. Doctors call these cancers HER2-positive.
HER2 testing is done on tumor tissue. If the test is positive, HER2-targeted treatments may work. If the cancer becomes resistant to one HER2 drug, another type of HER2-targeted therapy may still help—this is where Anbenitamab comes in.
How Is Anbenitamab Different from Older HER2 Drugs?
Most older HER2 medicines attach to one part of the HER2 protein. Anbenitamab (KN026) is different because it is a bispecific antibody. This means:
- It attaches to two different parts of HER2 at the same time
- It blocks HER2 signals more strongly
- It makes it harder for cancer cells to “escape” treatment
You can think of it like locking two doors instead of one, making it much harder for cancer cells to keep growing. Anbenitamab (KN026) also helps the immune system recognize and attack cancer cells, adding another layer of protection.
Why Was Anbenitamab Developed?
Many patients with HER2-positive gastric or GEJ cancer respond well to first-line HER2 therapy, but most cancers eventually stop responding. After that happens, treatment options are limited.
Anbenitamab (KN026) was designed to:
- Work after trastuzumab stops working
- Provide stronger HER2 blocking
- Offer benefit without the added risks of some chemotherapy-linked drugs
What Have Studies Shown So Far?
Large clinical studies have tested Anbenitamab (KN026) together with chemotherapy in patients whose HER2-positive gastric or GEJ cancer had already progressed after standard HER2 treatment.
The most important results were shared at a major international cancer meeting, the ESMO Congress, which highlights research that may change patient care.
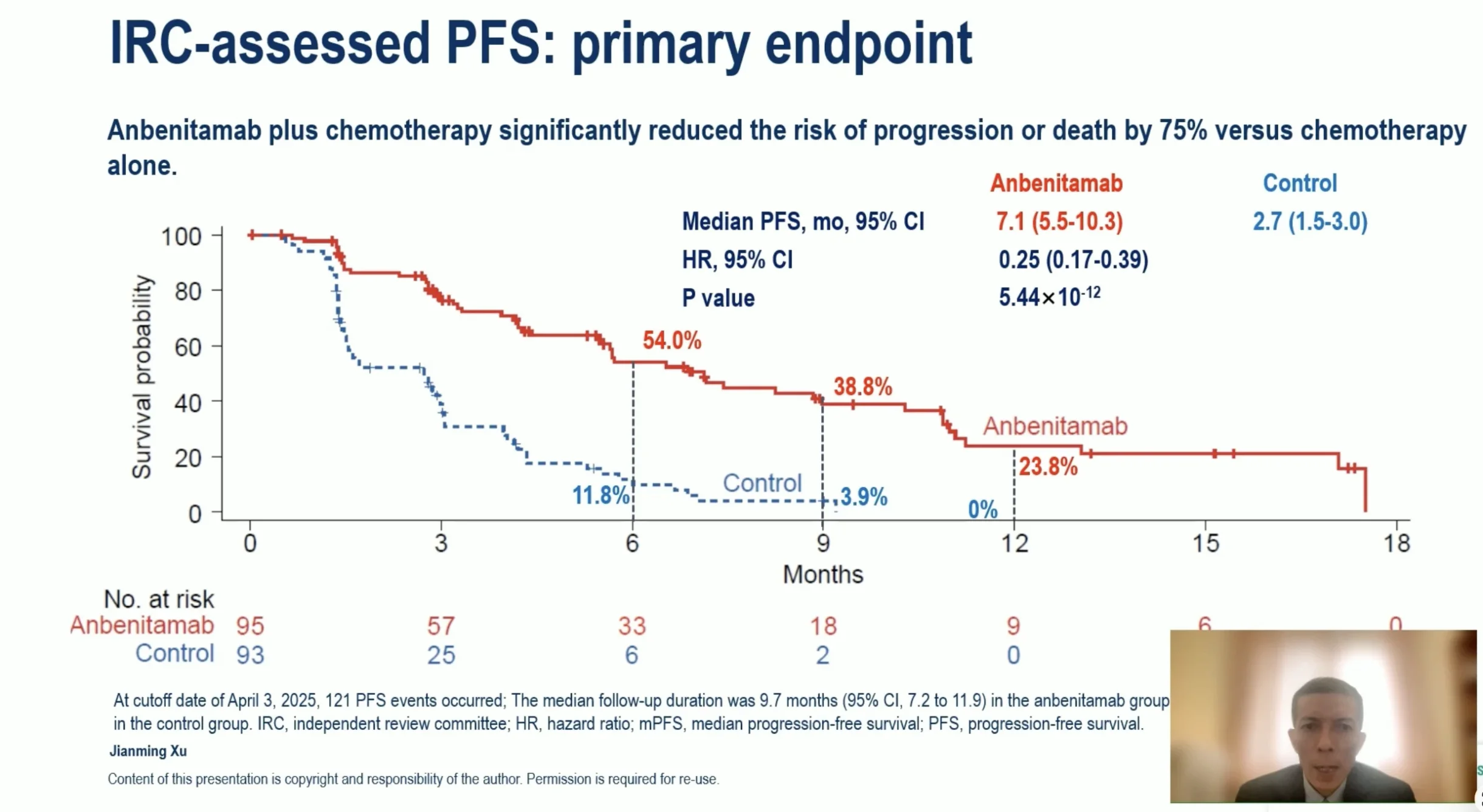
How Well Did It Work?
For patients receiving Anbenitamab (KN026) plus chemotherapy:
- Cancer was controlled for much longer compared with chemotherapy alone
- Patients lived significantly longer on average
- Tumors shrank in more than half of treated patients
- Responses lasted longer, not just briefly
These improvements were seen in patients with advanced disease who had already tried other treatments, which makes the results especially encouraging.
Is Anbenitamab Safe?
No cancer treatment is completely free of side effects, but Anbenitamab (KN026) has shown a manageable safety profile. Common side effects were mostly related to chemotherapy, such as:
- Low blood counts
- Fatigue
- Diarrhea
- Weakness
Importantly:
- Heart-related side effects were uncommon, which is reassuring for a HER2-targeted drug
- Serious side effects occurred at similar rates compared with standard treatment
- Many patients were able to stay on treatment longer because their cancer was better controlled
Your care team would monitor side effects closely and adjust treatment if needed.
Who Might Benefit from Anbenitamab?
Anbenitamab (KN026) may be considered for patients who:
- Have HER2-positive gastric or GEJ cancer
- Have already been treated with trastuzumab-based therapy
- Need new options after cancer progression
Studies are also ongoing in HER2-positive breast cancer, including earlier stages of disease.
Whether Anbenitamab (KN026) is right for you depends on:
- Your cancer type and HER2 status
- Previous treatments
- Overall health
- Local availability and regulatory approval
Is Anbenitamab Approved Yet?
Anbenitamab (KN026) is in advanced clinical development.
- It has received special regulatory designations in some regions to speed development
- Applications for approval are under review in certain countries
- More studies are ongoing to confirm benefits and expand its use
Your oncologist will know whether Anbenitamab (KN026) is available through clinical trials or approved programs in your area.

Read About 10 Most Promising Drugs Not Yet Approved in 2025 on OncoDaily
How Is Anbenitamab Given?
Anbenitamab (KN026) is given by intravenous infusion (through a vein), usually together with chemotherapy. Treatment schedules are carefully planned by oncology teams to balance effectiveness and safety.
Why This Matters for Patients
For many patients with HER2-positive gastric or GEJ cancer, treatment options after first-line therapy have been limited. Anbenitamab (KN026) represents a new way of targeting HER2, not by adding more chemotherapy, but by blocking the cancer signal more completely.
This approach offers hope for:
- Longer disease control
- Better tumor shrinkage
- Meaningful extension of survival
- A manageable safety profile
Questions You May Want to Ask Your Doctor
Is my cancer HER2-positive, and how was it tested?
Have I already received trastuzumab or similar HER2 therapy?
Could Anbenitamab (KN026) be an option for me now or in the future?
Are there clinical trials available?
What side effects should I expect compared with my current treatment?
A Reassuring Takeaway
Anbenitamab (KN026) is an important new HER2-targeted treatment designed for patients whose cancer needs stronger and smarter HER2 blocking. While research is still ongoing, results so far suggest that this medicine may help patients live longer and keep their cancer under control when other HER2 treatments have stopped working.
If you or a loved one has HER2-positive cancer, it’s reasonable to ask your care team whether Anbenitamab could be part of future treatment options.

Read Professional Version here Photo: Depositphotos


