For more than two decades, HER2 has stood as one of the most successfully targeted oncogenic drivers in solid tumors. From the introduction of trastuzumab to the advent of antibody–drug conjugates and small-molecule tyrosine kinase inhibitors, HER2-directed therapy has reshaped outcomes in breast and gastric cancers. Yet this progress has also exposed persistent vulnerabilities: incomplete pathway inhibition, HER2 heterogeneity, and near-universal resistance following sequential anti-HER2 therapies.
Anbenitamab was engineered specifically to address these limitations. Rather than adding incremental potency through cytotoxic payloads, Anbenitamab introduces structural innovation—a bispecific antibody capable of simultaneously binding two non-overlapping HER2 epitopes. This design enables deeper receptor blockade, more durable signal suppression, and sustained antitumor activity across heterogeneous tumor populations. As clinical data mature, Anbenitamab is emerging not merely as another HER2 agent, but as a platform-defining molecule with the potential to recalibrate HER2 treatment strategies across multiple disease settings.
Molecular Architecture and Bispecific Engineering
Anbenitamab (KN026) is a fully human IgG-like bispecific antibody developed using Alphamab Oncology’s proprietary CRIB (Charge Repulsion Induced Bispecific) platform. This Fc-based heterodimerization technology enables stable assembly of two distinct antigen-binding arms within a single antibody molecule while preserving favorable pharmacokinetic properties.
Structurally, Anbenitamab integrates trastuzumab-like and pertuzumab-like binding domains, allowing concurrent engagement of HER2 domain IV and domain II, respectively. This dual-epitope targeting is central to its mechanism, as it blocks both ligand-independent HER2 signaling and HER2 heterodimerization with other ErbB family members, particularly HER3. By preventing HER2–HER3 dimer formation, Anbenitamab disrupts downstream PI3K/AKT and MAPK signaling cascades that drive tumor proliferation, survival, and therapeutic resistance.
Importantly, Anbenitamab retains an intact Fc region, enabling antibody-dependent cellular cytotoxicity (ADCC). This immune effector function complements direct signal inhibition and may enhance tumor cell clearance, particularly in tumors with residual HER2 expression following prior therapy.
Mechanism of Action: From Receptor Engagement to Signal Extinction
At the cellular level, Anbenitamab achieves multi-layered HER2 suppression. Dual-epitope binding leads to enhanced receptor clustering, accelerated internalization, and lysosomal degradation of HER2. This reduces surface receptor density and prolongs pathway inhibition beyond what is achievable with monospecific antibodies.
Preclinical signaling studies have shown sustained suppression of phosphorylated HER2, AKT, and ERK in tumor models treated with Anbenitamab, even after drug washout. This prolonged signaling extinction distinguishes Anbenitamab from earlier HER2 antibodies and provides a mechanistic explanation for its observed clinical durability.
By integrating signal blockade, receptor downregulation, and immune-mediated cytotoxicity into a single molecular entity, Anbenitamab exemplifies a next-generation approach to antibody engineering in oncology.
Preclinical Validation Across HER2-Driven Tumors
Extensive preclinical evaluation established the translational rationale for Anbenitamab. In HER2-overexpressing breast and gastric cancer cell lines, Anbenitamab demonstrated superior growth inhibition compared with trastuzumab or pertuzumab alone. Notably, activity was preserved in trastuzumab-resistant models, supporting its ability to overcome adaptive resistance mechanisms.
In xenograft studies, Anbenitamab induced deeper and more durable tumor regression than conventional HER2 antibodies. Combination experiments revealed additive and synergistic effects when Anbenitamab was paired with chemotherapy or HER2-targeted tyrosine kinase inhibitors, further supporting its evaluation in multidrug regimens.
These data collectively positioned Anbenitamab as a clinically viable strategy for tumors that remain HER2-driven but have escaped earlier lines of HER2-directed therapy.
Early Clinical Development and Safety Characterization
Phase I clinical studies evaluated Anbenitamab in patients with advanced HER2-positive solid tumors, including heavily pretreated breast and gastric cancers. These studies established a manageable safety profile and favorable pharmacokinetics compatible with sustained HER2 blockade.
Infusion-related reactions were generally low grade and manageable. Importantly, cardiac toxicity—a historical concern with HER2-targeted therapies—was infrequent, with no signal of cumulative cardiomyopathy emerging during early development. These findings supported advancement into later-phase trials and combination strategies.
Clinical Validation in Advanced HER2-Positive Gastric and GEJ Cancer
While mechanistic sophistication is essential, the clinical relevance of Anbenitamab rests on its performance in tumors with high unmet need. This is most evident in HER2-positive gastric and gastroesophageal junction (GC/GEJ) cancers, where trastuzumab resistance, HER2 heterogeneity, and rapid disease progression have historically limited the durability of targeted approaches.
Clinical validation in this setting was provided by a pivotal phase III study evaluating Anbenitamab in combination with chemotherapy in patients with HER2-positive GC/GEJ who progressed after trastuzumab-based therapy. The first interim analysis, presented as a Late-Breaking Oral Presentation at the ESMO Congress 2025, represents the most compelling clinical evidence to date supporting the therapeutic potential of Anbenitamab.
Survival and Disease Control Outcomes
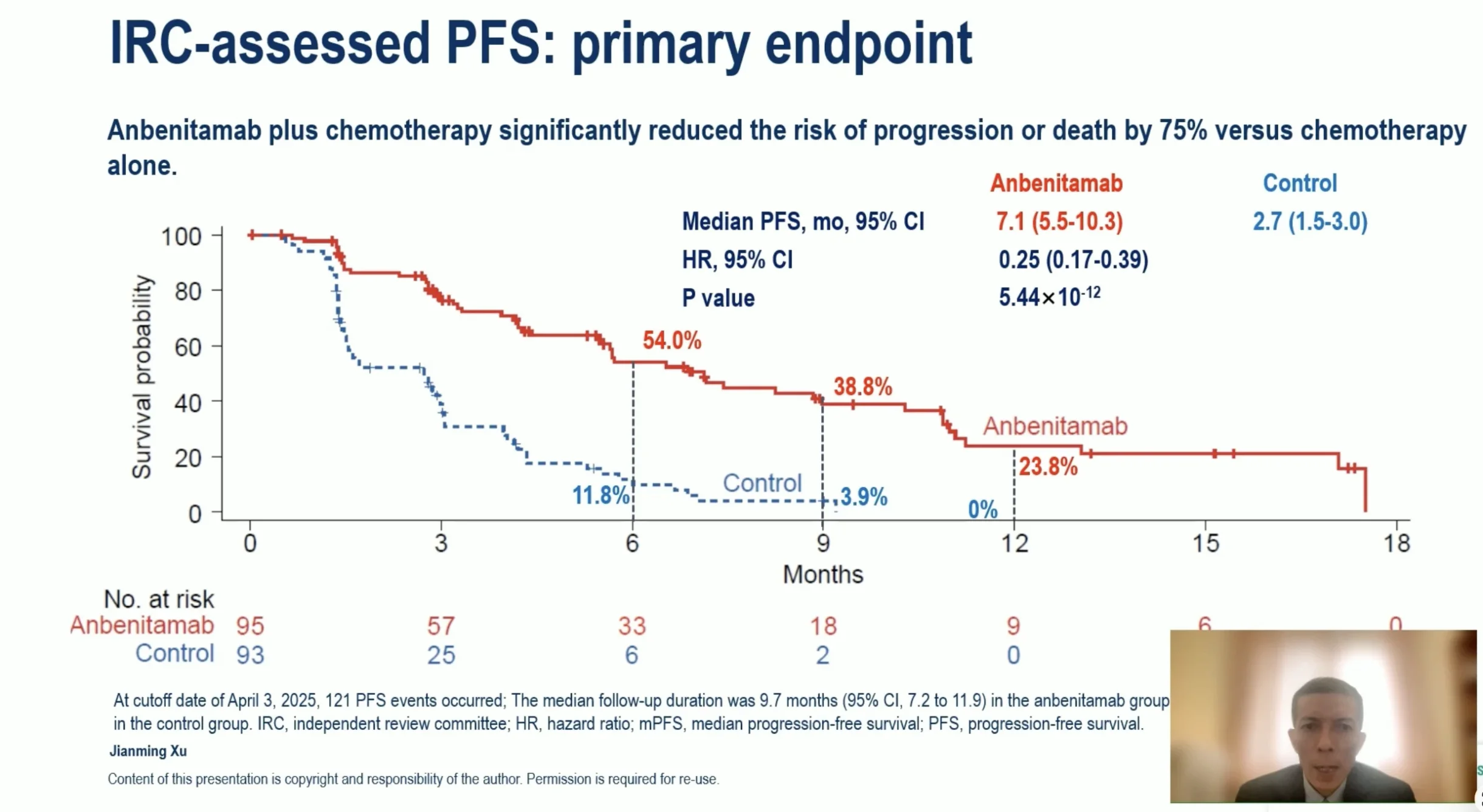
In a heavily pretreated population, Anbenitamab delivered exceptional improvements in both progression-free and overall survival. Median progression-free survival reached 7.1 months with Anbenitamab plus chemotherapy, compared with 2.7 months with chemotherapy alone, corresponding to a 75% reduction in the risk of disease progression or death. The statistical strength of this result was remarkable, underscoring the robustness of the observed benefit.
Overall survival outcomes further reinforced the clinical impact. Median overall survival reached 19.6 months in the Anbenitamab group versus 11.5 months in the control group, translating into a 71% reduction in the risk of death. Although survival data were not fully mature, early and sustained separation of survival curves suggests durable benefit.
Tumor Response and Durability
Anbenitamab also demonstrated high antitumor activity. Objective responses were observed in 55.8% of patients receiving Anbenitamab, compared with 10.8% in the control arm. Disease control was achieved in 80.0% versus 41.9%, respectively. Importantly, responses were durable, with a median duration of response of 8.2 months, compared with 2.9 months with chemotherapy alone.
Safety in Combination Therapy
The safety profile of Anbenitamab in combination with chemotherapy was consistent with expectations for advanced gastric cancer. Grade ≥3 adverse events were manageable and largely driven by chemotherapy backbone. Of particular importance, cardiotoxicity rates were low and identical (3.2%) in both treatment groups, reinforcing the cardiac safety of intensified HER2 blockade with Anbenitamab.
Positioning Anbenitamab in the Evolving HER2 Landscape
The HER2 therapeutic landscape is increasingly complex, with antibody–drug conjugates such as trastuzumab deruxtecan redefining efficacy benchmarks in some settings. Within this environment, Anbenitamab occupies a distinct and strategically important niche as a non-conjugated bispecific antibody capable of delivering deep pathway inhibition without cytotoxic payloads.
This differentiation may be particularly relevant for combination strategies, earlier lines of therapy, and patients in whom toxicity limits the use of ADCs. Moreover, Anbenitamab’s activity in trastuzumab-refractory gastric cancer highlights the value of structural antibody innovation as a complement to payload-driven approaches.
Regulatory Milestones and Ongoing Development
Reflecting its clinical promise, Anbenitamab has received Breakthrough Therapy Designation from China’s National Medical Products Administration for trastuzumab-refractory HER2-positive gastric cancer and Orphan Drug Designation from the U.S. Food and Drug Administration for HER2-positive or HER2-low gastric cancer. In September 2025, the first New Drug Application for Anbenitamab in combination with chemotherapy was accepted by the NMPA.
Beyond gastric cancer, Anbenitamab continues to be evaluated in HER2-positive breast cancer, including first-line and neoadjuvant settings. In parallel, next-generation antibody–drug conjugates built on the KN026 backbone are advancing, further extending the therapeutic reach of this bispecific platform.

Read About 1o Most Promising Drugs Not Yet Approved in 2025 on OncoDaily


