
Relatlimab : What Patients Need To Know?
Relatlimab is a novel immunotherapy drug developed by Bristol Myers Squibb (BMS) that targets the lymphocyte activation gene-3 (LAG-3). This innovative checkpoint inhibitor is part of a combination therapy with nivolumab (Opdualag) and was approved by the U.S. Food and Drug Administration (FDA) in March 2022. It is the first LAG-3-blocking combination therapy for unresectable or metastatic melanoma in adults and pediatric patients (aged 12 years and older). By enhancing T-cell activation, relatlimab offers new hope to patients battling advanced melanoma.
What Is Relatlimab and How Does It Work?
Relatlimab is a human monoclonal antibody that blocks the LAG-3 protein, an inhibitory receptor on T cells that dampens immune responses. LAG-3 is often overexpressed in cancer patients, suppressing the immune system’s ability to attack tumors. By inhibiting LAG-3, relatlimab restores T-cell function, promoting a more robust anti-tumor response.
When combined with nivolumab, a PD-1 inhibitor, the therapy targets two key immune checkpoint pathways simultaneously, leading to a more powerful and sustained immune response against cancer cells. This dual blockade approach has shown significant improvements in delaying disease progression in melanoma patients.
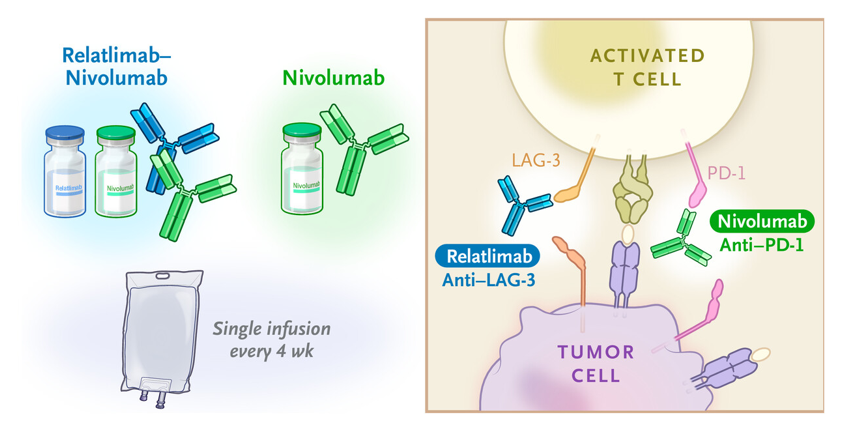
How Is Relatlimab Administered?
Relatlimab is given as an intravenous (IV) infusion, meaning it is delivered directly into the bloodstream through a vein. It is administered in combination with nivolumab (Opdualag) every four weeks. The infusion itself takes about 30-60 minutes, and patients are monitored during and after treatment for any reactions. Unlike chemotherapy, which directly attacks cancer cells, relatlimab helps the immune system recognize and destroy cancer more effectively.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before relatlimab was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.
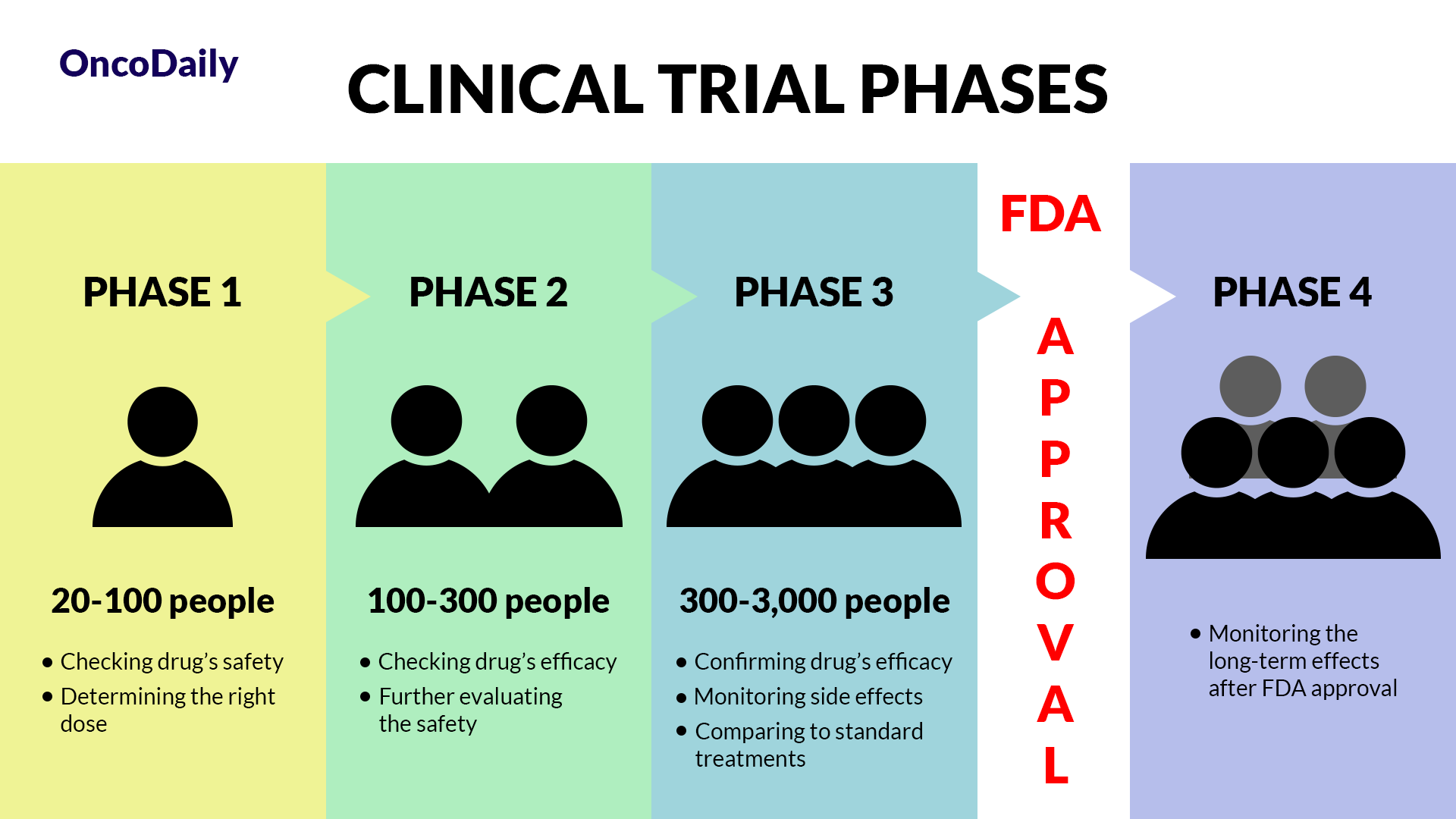
What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
What Cancers Does Relatlimab Treat?
Currently, relatlimab (as part of Opdualag) is FDA-approved for the treatment of:
- Unresectable or metastatic melanoma (for adults and pediatric patients aged 12 and older).
Research is ongoing to determine its effectiveness in other cancers, including lung cancer, colorectal cancer, and hepatocellular carcinoma (HCC). Clinical trials are evaluating whether the LAG-3/PD-1 combination can provide benefits in additional tumor types.
Efficacy and Results from Clinical Trials
The approval of relatlimab was based on the pivotal RELATIVITY-047 trial, which compared Opdualag (relatlimab + nivolumab) to nivolumab alone in patients with advanced melanoma. Key findings included:
- Progression-Free Survival (PFS): 10.1 months with Opdualag vs. 4.6 months with nivolumab alone.
- Overall Response Rate (ORR): Higher with Opdualag, indicating more patients experienced tumor shrinkage.
- Durability of Response: The dual therapy demonstrated prolonged disease control compared to PD-1 inhibition alone.
These results marked a major advancement in melanoma treatment, supporting the approval of relatlimab.
What Other Trials Are Ongoing?
Several ongoing clinical trials are exploring the use of relatlimab in other cancers, including:
- Non-Small Cell Lung Cancer (NSCLC) – Evaluating efficacy in early-stage and metastatic disease.
- Triple-Negative Breast Cancer (TNBC) – Assessing its role in neoadjuvant therapy.
- Colorectal Cancer (CRC) – Investigating potential benefits in microsatellite stable (MSS) tumors.
- Hepatocellular Carcinoma (HCC) – Testing its use in combination with bevacizumab.
- Urothelial Bladder Cancer (TURANDORELA trial) – Studying pre-operative nivolumab with or without relatlimab.
FDA Approval and Patient Access
FDA approval means that Opdualag is now available for eligible melanoma patients. With this approval, patients have access to a novel treatment option that significantly improves disease control. Insurance coverage and reimbursement policies vary, so patients should discuss options with their healthcare providers.
What Can You Expect During Treatment?
- Administration: Opdualag is given as an intravenous (IV) infusion every four weeks.
- Duration: The infusion typically lasts 30-60 minutes.
- Monitoring: Patients are observed for any immediate reactions and may require blood tests to assess liver function and immune response.
- Pre-Medication: Some patients receive medications to reduce the risk of infusion reactions.
Side Effects and Management
Like all immunotherapies, relatlimab comes with potential side effects. Common ones include:
- Fatigue
- Musculoskeletal pain
- Rash and pruritus (itching)
- Diarrhea

Serious immune-related side effects may include
- Pneumonitis (lung inflammation) – Symptoms include shortness of breath and persistent cough.
- Colitis (inflammation of the colon) – May cause diarrhea and abdominal pain.
- Hepatitis (liver inflammation) – Requires regular liver function monitoring.
- Endocrinopathies (hormonal imbalances) – Can lead to adrenal insufficiency or thyroid dysfunction.
Patients should report any unusual symptoms to their doctor immediately to ensure timely management.
What to Expect Long-Term
While relatlimab is not a cure, it significantly extends disease control and progression-free survival in melanoma patients. Some individuals experience long-lasting remission, while others may need additional treatments over time. Researchers are actively investigating how to optimize the duration of response and expand its use in other cancers.
What Should You Avoid During Treatment?
- Certain Medications: Immunosuppressants (e.g., corticosteroids) may reduce effectiveness.
- Live Vaccines: Patients should avoid live vaccines during treatment.
- Alcohol and Smoking: These can weaken the immune system and worsen side effects.
Real-Life Effectiveness and Future Outlook
Real-world data suggest that relatlimab performs similarly to clinical trial results. Patients benefit from prolonged progression-free survival and improved quality of life. Future research will focus on:
- Expanding its use to other cancers
- Combining it with other immunotherapies or targeted therapies
- Refining patient selection based on biomarkers to enhance effectiveness
Relatlimab, in combination with nivolumab, represents a major breakthrough in melanoma treatment and is being explored in several other cancers. With its unique mechanism of action targeting LAG-3, it offers new hope for patients needing more effective immunotherapy options. If you or a loved one are considering this treatment, consult with an oncologist to determine whether Opdualag may be right for you.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
