Relatlimab, a human monoclonal antibody targeting LAG-3 (lymphocyte activation gene-3), is an innovative checkpoint inhibitor developed by Bristol Myers Squibb (BMS). Approved by the FDA in March 2022, relatlimab + nivolumab (Opdualag) is the first LAG-3-blocking combination therapy indicated for unresectable or metastatic melanoma in adults and pediatric patients (≥12 years old). By blocking LAG-3, relatlimab enhances T-cell activation and anti-tumor immunity, complementing the PD-1 inhibition of nivolumab. The pivotal RELATIVITY-047 trial demonstrated a 10.1-month median progression-free survival (PFS) with the dual therapy, compared to 4.6 months with nivolumab alone, marking a paradigm shift in melanoma immunotherapy. Ongoing clinical trials are evaluating relatlimab’s potential in lung cancer, colorectal cancer, and hepatocellular carcinoma (HCC), making it a key player in next-generation immuno-oncology treatments.
What Drug Is Relatlimab?
Relatlimab is a human monoclonal antibody that functions as an immunotherapy drug. it targets the lymphocyte activation gene-3 (LAG-3) protein, an inhibitory receptor on T cells. In March 2022, the U.S. Food and Drug Administration (FDA) approved a fixed-dose combination of relatlimab and nivolumab, marketed as Opdualag, for the treatment of adult and pediatric patients aged 12 years and older with unresectable or metastatic melanoma. This approval marked the first LAG-3-blocking antibody combination therapy, offering a novel approach distinct from traditional chemotherapy.
Which company produced Relatlimab?
Relatlimab is produced by Bristol Myers Squibb (BMS), a global biopharmaceutical company based in New York, USA. BMS specializes in innovative therapies for oncology, hematology, immunology, and cardiovascular diseases. It has been a pioneer in immuno-oncology, developing checkpoint inhibitors like nivolumab (Opdivo), ipilimumab (Yervoy), and relatlimab, which target different immune pathways to enhance anti-cancer responses.
Relatlimab is co-formulated with nivolumab as Opdualag. It is available in two dosage strengths:
- 480 mg nivolumab + 160 mg relatlimab per 20 mL vial (24 mg/mL + 8 mg/mL)-Fixed dose
- 240 mg nivolumab + 80 mg relatlimab per 20 mL vial (12 mg/mL + 4 mg/mL)
The 240 mg/80 mg dose is used for patients requiring a lower dose, often based on weight or specific clinical considerations. Both formulations are administered as an intravenous infusion every 4 weeks.
The patent for Opdualag, a combination therapy of nivolumab and relatlimab developed by Bristol Myers Squibb, is projected to expire in 2028. This expiration date is based on disclosures by the brand-side company in response to potential biosimilar entries.
How Does Relatlimab Work?
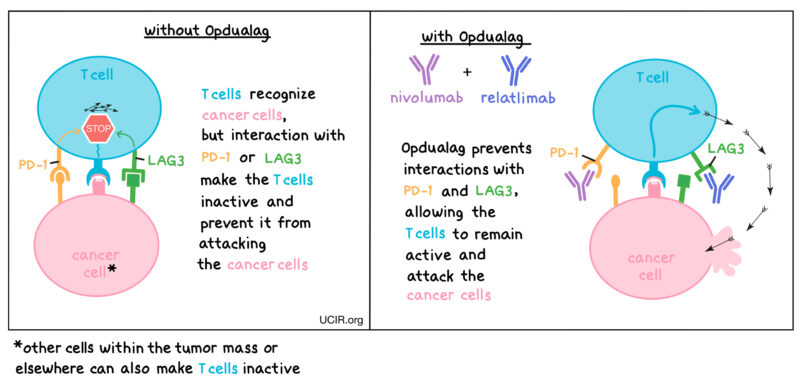
Relatlimab operates by inhibiting the LAG-3 pathway. LAG-3 is an immune checkpoint receptor that, when engaged, downregulates T-cell activity, allowing cancer cells to evade immune detection. By binding to LAG-3, relatlimab blocks its interaction with ligands, including MHC II, reducing LAG-3-mediated inhibition of the immune response. This action promotes T cell proliferation and cytokine secretion, enhancing the body’s ability to attack cancer cells. When combined with nivolumab, a PD-1 inhibitor, the dual blockade of LAG-3 and PD-1 pathways results in increased T cell activity compared to either antibody alone.
You can read more about Opdivo (Nivolumab) Uses in Cancer, Side Effects, Dosages on OncoDaily.
What Cancers Is Relatlimab Approved to Treat?
As of its approval, the fixed-dose combination of relatlimab and nivolumab (Opdualag) is indicated for treating unresectable or metastatic melanoma in adults and pediatric patients aged 12 years and older.
The approval was based on the results of the phase 2/3 RELATIVITY-047 trial, which demonstrated that patients receiving the combination therapy had a median progression-free survival (PFS) of 10.1 months, compared to 4.6 months for those treated with nivolumab alone. This significant improvement underscores the efficacy of the combination in delaying disease progression.
Given that the approved version of Relatlimab is combined with Nivolumab, we will discuss the side effects, dosage, treatment expectations, long-term effectiveness, and ongoing trials for the combination version marked as Opdualag.
What Are the Side Effects of Opdualag?
Common side effects include musculoskeletal pain, fatigue, rash, pruritus, and diarrhea. Serious immune-related adverse events have been reported, such as adrenal insufficiency, anemia, colitis, pneumonia, acute myocardial infarction, back pain, diarrhea, myocarditis, and pneumonitis.
Patients undergoing cancer treatment may experience side effects like pneumonitis, fatigue, and cough. Pneumonitis, an inflammation of lung tissue, can present as a new or worsening cough, chest pain, or shortness of breath. Early detection is crucial; patients should promptly report respiratory symptoms to their healthcare provider. Management may involve corticosteroids and temporary or permanent discontinuation of therapy, depending on severity.
Liver-specific immune-related side effects, such as hepatitis or elevated liver enzymes, have also been observed. Regular liver function tests are essential to monitor for hepatotoxicity. Symptoms like fatigue, jaundice, or abdominal pain should be reported immediately. Management strategies include corticosteroid administration and, in severe cases, discontinuation of treatment.

In rare instances, life-threatening side effects like cytokine release syndrome or severe organ-specific toxicities can occur. Patients should be vigilant for symptoms such as high fever, difficulty breathing, or significant organ dysfunction. Immediate medical attention is imperative, and treatment may require hospitalization, intensive supportive care, and adjustments to therapy.
Opdualag during pregnancy
The safety of Obdualag during pregnancy is not established. Based on animal studies and their mechanism of action, these drugs may cause fetal harm. Women of reproductive potential should use effective contraception during treatment and for at least five months after discontinuation. While there are no controlled human studies, animal data suggest an increased risk of spontaneous abortion and neonatal death. In Australia, this combination is classified as Pregnancy Category D, indicating potential fetal risks. The FDA has not assigned a specific pregnancy category.
What are the recommended dosage and storage requirements for Opdualag?
For the treatment of unresectable or metastatic melanoma, the FDA recommends a fixed-dose combination of 480 mg nivolumab and 160 mg relatlimab administered intravenously every 4 weeks. This regimen applies to both adult and pediatric patients aged 12 years and older who weigh at least 40 kg. Currently, no dosage adjustments are established for patients weighing less than 40 kg.
The prepared solution can be stored at room temperature and light for up to eight hours before it must be discarded. If refrigerated at 2°C to 8°C (36°F–46°F) and protected from light, it remains usable for up to 24 hours, including the time needed to reach room temperature before infusion. To maintain stability, the solution should not be frozen or shaken.
What to Expect During Opdualag Therapy?
Before initiating therapy, patients undergo comprehensive assessments, including blood tests and imaging studies, to establish baselines and evaluate overall health. It’s crucial to inform healthcare providers about existing medical conditions, allergies, and all medications or supplements being taken to prevent potential interactions.
During the infusion, which typically lasts 30 to 60 minutes, patients are monitored for immediate adverse reactions.
After the infusion, regular follow-up appointments are essential. These may include blood tests and imaging scans to monitor treatment progress and detect potential side effects. Patients are encouraged to maintain open communication with their healthcare team, reporting any new or worsening symptoms promptly. Managing physical, emotional, and social well-being during therapy is vital. Engaging in light physical activity, adhering to a balanced diet, managing fatigue through adequate rest, and seeking support from counseling or community groups can enhance the quality of life during treatment.
What to Avoid During Opdualag Treatment?
Certain medications, such as corticosteroids and other immunosuppressants, may interfere with Opdualag’s effectiveness. It’s essential to inform healthcare providers about all current medications and supplements to avoid potential interactions. While no specific foods are known to cause adverse reactions with Opdualag, maintaining a balanced diet supports overall health. Consulting with a nutritionist can provide personalized dietary recommendations during treatment. Alcohol and smoking can weaken the immune system and potentially exacerbate side effects. Reducing or eliminating these habits is advisable. Healthcare providers can offer resources and support to assist in cessation efforts.
Opdualag’s Effectiveness Over Time
Clinical trials have demonstrated that the combination of Relatlimab and Nivolumab can improve progression-free survival in patients with advanced melanoma.
Some studies have failed to meet their primary efficacy endpoints. Trials like FRACTION-GC (gastric cancer), FRACTION-RCC (renal cell carcinoma), and the nivolumab + relatlimab study in MSS colorectal cancer demonstrated manageable safety but did not show significant improvements in overall survival (OS) or progression-free survival (PFS).
The Phase II study of nivolumab and relatlimab (Obdualag) in MSS advanced colorectal cancer (CRC) enrolled 59 patients. While the combination was well tolerated, the objective response rate (ORR) was below 20%. However, patients with lung-only metastases showed a 33% partial response and a 50% clinical benefit rate. In the FRACTION-RCC trial, nivolumab + relatlimab had an ORR of 30%, while nivolumab + ipilimumab had 20%. Both regimens demonstrated activity, with nivolumab + relatlimab showing fewer Grade 3-4 adverse events (13% vs. 33%).
The phase II study enrolled 60 treatment-naïve patients with resectable NSCLC, randomized to preoperative nivolumab with or without relatlimab. The primary endpoint, surgical feasibility within 43 days, was met by all. Curative resection was achieved in 95%, with major pathological responses in 27% (nivolumab) vs. 30% (nivolumab + relatlimab) and radiographic responses in 10% vs. 27%, respectively. At the 12-month follow-up, disease-free survival was 89% (nivolumab) vs. 93% (nivolumab + relatlimab), and overall survival was 93% vs. 100%.
At ESMO Congress 2024, results from the BELLINI trial (cohorts C and D) were presented, evaluating neoadjuvant nivolumab with either ipilimumab or relatlimab in triple-negative breast cancer (TNBC) patients with high tumor-infiltrating lymphocytes (TILs). Patients received treatment for 6–8 weeks before surgery. The pathological complete response (pCR) rate was 33% for nivolumab + ipilimumab and 47% for nivolumab + relatlimab, meeting the expansion criteria for further study. However, immune-related adverse events (IR-AEs), particularly endocrinopathies, were noted, especially with nivolumab + low-dose ipilimumab.
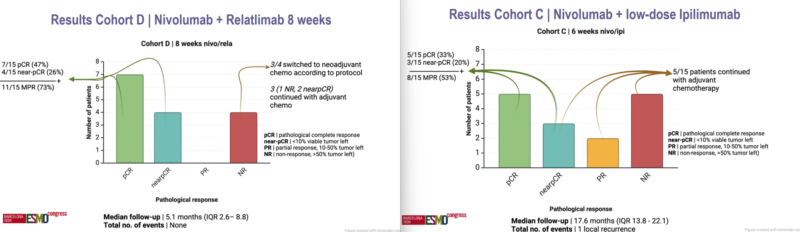
Future research will explore long-term quality of life, response durability, and reasons for non-response despite high TIL levels.
At ESMO Congress 2024, results from the NICHE-3 trial were presented, evaluating neoadjuvant nivolumab plus relatlimab in patients with MMR-deficient (dMMR) colon cancer. This phase 2, single-arm study aimed to assess pathologic response rates and safety in non-metastatic, treatment-naïve patients. The primary endpoint was met, with 97% of patients achieving a pathologic response, including 92% major pathologic responses (≤10% residual tumor) and 68% complete responses (0% residual tumor). Surgical feasibility was high, with 100% tumor-free resection margins, and only 5% of patients had surgical delays. Immune-related adverse events (irAEs) were observed in 80% of patients, but grade 3–4 events were low (10%). At a median follow-up of 8 months, 98% remained disease-free, and all patients were alive. These findings further support neoadjuvant immune checkpoint blockade (ICB) as a standard-of-care in dMMR colon cancer and warrant larger multinational studies to optimize treatment strategies.
Ongoing clinical trials with Relatlimab
Here are ongoing clinical trials evaluating relatlimab, often in combination with nivolumab, marked as Obdualag, across various cancer types beyond melanoma. These trials aim to assess its efficacy and safety in cancers such as non-small cell lung cancer (NSCLC), head and neck cancers, and metastatic uveal melanoma (UM). Some studies focus on first-line treatment, while others explore its role in previously treated patients. Another trial is testing triplet therapy (nivolumab, relatlimab, and bevacizumab) in untreated advanced hepatocellular carcinoma (HCC).
The TURANDORELA trial (NCT06237920) is a phase 2 study evaluating pre-operative nivolumab with or without relatlimab in 90 patients with stage II–IIIa urothelial bladder cancer. Patients receive two cycles of treatment every 28 days, with response assessed via cystoscopy, mpMRI, and CT scan. The primary endpoint is pathological complete response (pT0N0 or pTisN0) at cystectomy. Secondary outcomes include treatment feasibility, safety, survival, and event-free survival. Follow-ups at 6, 12, and 24 months will assess long-term effects, QoL, and adverse events.
Written by Mariam Khachatryan, MD
FAQ
What is Relatlimab, and how does it work?
Relatlimab is a monoclonal antibody that blocks the LAG-3 protein, helping the immune system recognize and attack cancer cells more effectively.
What is the approved dosage of Relatlimab in combination with Nivolumab?
The approved dose of Opdualag (Relatlimab + Nivolumab) is a fixed-dose combination of 160 mg Relatlimab and 480 mg Nivolumab, given as an intravenous infusion every four weeks.
What are the common side effects of Opdualag?
Common side effects include fatigue, rash, itching, diarrhea, muscle and joint pain, and increased liver enzymes. Severe immune-related side effects can also occur.
How effective is Opdualag in treating melanoma?
Clinical trials have shown that Opdualag improves progression-free survival in patients with advanced melanoma compared to Nivolumab alone.
How should Opdualag be stored?
Opdualag should be stored in a refrigerator (2°C–8°C) and protected from light. It should not be frozen or shaken before use.
What should patients expect during Opdualag treatment?
Patients may experience mild to moderate side effects, with regular monitoring required to manage any severe immune-related reactions.
Can Opdualag be used as a first-line treatment for melanoma?
Yes, Opdualag is FDA-approved for first-line treatment of unresectable or metastatic melanoma in adults and children over 12 years old.
How does Relatlimab differ from other immune checkpoint inhibitors?
Unlike PD-1 or CTLA-4 inhibitors, Relatlimab targets LAG-3, another immune checkpoint that suppresses T-cell activity in cancer.
Who should avoid Opdualag treatment?
Patients with severe autoimmune diseases, organ transplants, or a history of serious immune-related side effects should consult their doctor before using Opdualag.


