Phase 3 VERITAC-2 trial (NCT05654623) results were announced by Arvinas and Pfizer. evaluating vepdegestrant, an investigational oral PROTAC degrader, against fulvestrant in patients with ER+/HER2- advanced or metastatic breast cancer following disease progression on CDK4/6 inhibitors and endocrine therapy.

VERITAC-2 successfully met its primary endpoint in the estrogen receptor 1-mutant (ESR1m) population, showing a statistically significant and clinically relevant improvement in progression-free survival (PFS). Additionally, vepdegestrant is the first PROTAC degrader to exhibit clinical efficacy in a Phase 3 trial.
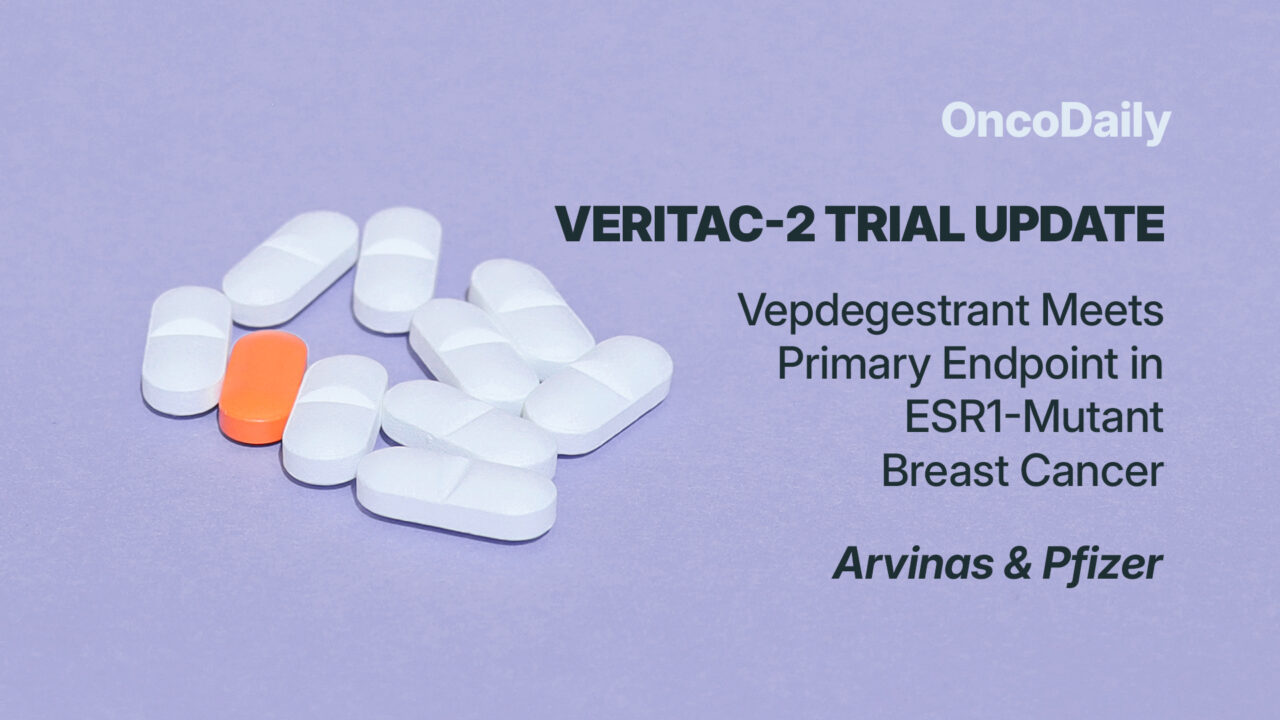
What Drug is Vepdegestrant and How Does it Work?
Vepdegestrant is an investigational oral Proteolysis Targeting Chimera (PROTAC) degrader designed to specifically target and degrade the estrogen receptor (ER) in ER-positive/HER2-negative (ER+/HER2-) advanced or metastatic breast cancer. It is being co-developed by Arvinas and Pfizer.
Unlike traditional endocrine therapies that inhibit ER signaling, PROTAC degraders use the body’s natural protein disposal system to selectively eliminate the estrogen receptor (ER) rather than just blocking its activity. This degradation approach aims to overcome resistance mechanisms, including ESR1 mutations, which commonly arise in patients previously treated with endocrine therapy and CDK4/6 inhibitors.
What is Standard Treatment for Advanced Breast Cancer Beyond Vepdegestrant?
The standard treatment for ER+/HER2- breast cancer typically involves the use of aromatase inhibitors (AIs) combined with CDK4/6 inhibitors. Aromatase inhibitors, such as letrozole, anastrozole, and exemestane, work by blocking the enzyme aromatase, which is responsible for converting androgens into estrogen in postmenopausal women. By reducing estrogen levels, AIs effectively limit the hormonal stimulation of ER-positive breast cancer cells, slowing tumor growth.
CDK4/6 inhibitors, such as palbociclib, ribociclib, and abemaciclib, complement this treatment by inhibiting cyclin-dependent kinases (CDKs) 4 and 6, proteins that regulate the cell cycle. By blocking these proteins, CDK4/6 inhibitors prevent cancer cells from progressing through the cell cycle, thereby inhibiting cell division and proliferation.
Development of Endocrine Therapy (ET) Resistance
Despite the initial effectiveness of these therapies, endocrine therapy (ET) resistance often develops over time. One of the key mechanisms driving this resistance is the emergence of ESR1 mutations. ESR1 mutations occur in around 40% of patients who have received long-term treatment with aromatase inhibitors. These mutations alter the estrogen receptor, making it constitutively active and capable of driving tumor growth even in the absence of estrogen. As a result, the cancer cells become less responsive to therapies that block estrogen signaling, including AIs, leading to disease progression.
How Vepdegestrant Compares with other ESR1 inhibotrs?
Vepdegestrant is an investigational oral PROTAC estrogen receptor (ER) degrader, designed to target and degrade the estrogen receptor, including ESR1 mutations, which are often responsible for resistance to traditional endocrine therapies. Compared to other ESR1 inhibitors like fulvestrant and elacestrant, vepdegestrant offers a more complete degradation of the estrogen receptor, which may provide superior efficacy, especially in ESR1-mutant breast cancer. Fulvestrant, an established selective estrogen receptor degrader (SERD), is administered intramuscularly and promotes partial degradation of the ER, limiting its effectiveness in patients with ESR1 mutations.
Elacestrant, a newer oral SERD, has shown better clinical efficacy than fulvestrant, particularly in patients with ESR1 mutations, offering a convenient alternative for oral administration. While vepdegestrant is still undergoing clinical trials, early data shows promising results, suggesting it could overcome resistance mechanisms more effectively than fulvestrant and elacestrant. Vepdegestrant’s novel mechanism as a PROTAC degrader positions it as a potentially stronger option for treating ESR1-mutant breast cancer, with the added benefit of oral administration, improving patient adherence.
What is VERITAC-2 trial?
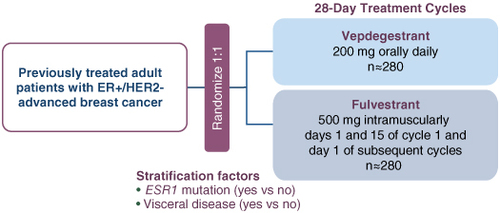
VERITAC-2 (NCT05654623): A Phase 3 trial evaluating vepdegestrant monotherapy versus fulvestrant in ER+/HER2- advanced/metastatic breast cancer after prior CDK4/6 inhibitor and endocrine therapy failure.
Background
Estrogen receptor-positive (ER+)/HER2-negative (HER2-) breast cancer is the most prevalent subtype of breast cancer, accounting for approximately 70-80% of all breast cancer diagnoses. This subtype is primarily driven by estrogen receptor (ER) signaling, which plays a critical role in promoting tumor growth. Estrogen binds to the ER on cancer cells, triggering a cascade of events that stimulates cell division and proliferation. As a result, therapies targeting ER signaling are cornerstone treatments for ER+/HER2- breast cancer.
Study Design and Methods
VERITAC-2 is a randomized, global Phase 3 study enrolling 624 patients across 26 countries. Participants were randomized to receive either oral vepdegestrant (daily, continuous dosing) or intramuscular fulvestrant (standard schedule). The primary endpoint was progression-free survival (PFS) in both the intent-to-treat (ITT) and ESR1-mutant (ESR1m) populations, as assessed by blinded independent central review (BICR). Overall survival (OS) is a key secondary endpoint, but data remain immature.
Key Findings
- The trial met its primary endpoint in the ESR1m population, demonstrating a statistically significant and clinically meaningful improvement in PFS over fulvestrant.
- The observed benefit exceeded the pre-specified target hazard ratio (HR) of 0.60 in ESR1m patients.
- In the overall ITT population, the improvement in PFS did not reach statistical significance.
- OS data remain immature, with less than 25% of the required events observed.
- Safety profile: Vepdegestrant was well tolerated, with a safety profile consistent with prior studies.
Results
Primary Endpoint Met: Significant improvement in progression-free survival (PFS) in patients with ESR1 mutations.
- Hazard Ratio (HR) < 0.60, exceeding the predefined threshold.
- Did not reach statistical significance in the intent-to-treat (ITT) population.
- Overall survival data are immature, with ongoing follow-up.
- Well-tolerated safety profile, consistent with prior studies.
Clinical Significance of VERITAC-2 Trial
Vepdegestrant is the first PROTAC degrader to demonstrate clinical benefit in a Phase 3 trial, supporting its potential as a first-in-class therapy for ESR1m metastatic breast cancer. Given the high prevalence of ESR1 mutations (~40% of second-line cases) and the limited treatment options post-CDK4/6 inhibitor therapy, these findings highlight a potential new standard of care.
What to Expect Next?
- Full data will be presented in 2025.
- Results will be submitted to global regulatory agencies to support potential regulatory approval.
About Arvinas and Pfizer Oncology
Arvinas (Nasdaq: ARVN) is a clinical-stage biotechnology company focused on developing innovative therapies to treat life-threatening diseases. The company utilizes its PROTAC (PROteolysis TArgeting Chimera) platform to create protein degradation therapies that leverage the body’s natural system to selectively eliminate disease-causing proteins. Arvinas is advancing several investigational drugs, including vepdegestrant, targeting the estrogen receptor in ER+/HER2- metastatic breast cancer; ARV-393, targeting BCL6 for non-Hodgkin lymphoma; and ARV-102, targeting LRRK2 for neurodegenerative disorders. Based in New Haven, Connecticut, Arvinas is leading the charge in protein degradation research to address unmet medical needs.
Pfizer Oncology is a leader in cancer care, with a diverse portfolio and pipeline focused on breakthrough therapies for various cancers. With expertise in small molecules, antibody-drug conjugates (ADCs), and bispecific antibodies, Pfizer Oncology targets cancer from multiple angles. Their commitment to delivering transformative treatments spans breast cancer, genitourinary cancer, hematology-oncology, and lung cancer. Pfizer Oncology is driven by a passion for science and is dedicated to accelerating new therapies to help cancer patients live better, longer lives.
You Can Read the Press Release here
Written by Sona Karamyan, MD