The HARMONi-2 study, a randomized phase 3 trial conducted in China, compared ivonescimab with pembrolizumab as first-line treatment for advanced PD-L1-positive non-small cell lung cancer (NSCLC). Ivonescimab demonstrated a significant improvement in progression-free survival (PFS), reducing the risk of disease progression or death by 49%, with benefits observed across PD-L1 expression subgroups and both squamous and non-squamous histologies.
Title: Ivonescimab versus pembrolizumab for PD-L1-positive non-small cell lung cancer (HARMONi-2): a randomised, double-blind, phase 3 study in China
Authors
Prof Anwen Xiong, PhD, Prof Lei Wang, PhD, Prof Jianhua Chen, MS, Prof Lin Wu, MD, Prof Baogang Liu, PhD, Prof Jun Yao, MD, Prof Hua Zhong, PhD, Prof Jie Li, MS, Prof Ying Cheng, MD, Prof Yulan Sun, PhD, Prof Hui Ge, PhD, Prof Jifang Yao, PhD, Prof Qin Shi, BS, Prof Ming Zhou, PhD, Bolin Chen, MD, Prof Zhengxiang Han, MD, Prof Jinliang Wang, MD, Prof Qing Bu, MD, Prof Yanqiu Zhao, MDo ∙ Prof Junqiang Chen, BSp ∙ Prof Ligong Nie, MSq ∙ Prof Gaofeng Li, MD, Prof Xingya Li, MD, Xinmin Yu, MD, Prof Yinghua Ji, MS, Prof Daqiang Sun, MD, Prof Xiaohong Ai, PhD, Prof Qian Chu, PhD, Prof Yu Lin, BS, Jiqing Hao, MD, Prof Dingzhi Huang, MD, Prof Chengzhi Zhou, PhD, Prof Jinlu Shan, MD, Prof Hongzhong Yang, MD, Prof Xuewen Liu, MD, Prof Jing Wang, PhD, Prof Yanhong Shang, MS, Prof Xiaodong Mei, PhD, Jie Yang, PhD, Dongmei Lu, MS, Mingxiu Hu, PhD, Zhongmin Maxwell Wang, PhD, Baiyong Li, PhD, Michelle Xia, PhD, rof Caicun Zhou, MD
Published in Lancet, March 2025
Background
Lung cancer remains the leading cause of cancer-related deaths worldwide, with non-small cell lung cancer (NSCLC) accounting for approximately 85% of cases. For patients with advanced NSCLC lacking actionable driver mutations, immune checkpoint inhibitors targeting PD-1 or PD-L1 have become the cornerstone of treatment. However, pembrolizumab, a widely used PD-1 inhibitor, has demonstrated varying efficacy depending on PD-L1 tumor proportion score (TPS), with the greatest benefit seen in patients with TPS ≥50%. The HARMONi-2 trial aimed to evaluate whether ivonescimab, a novel bispecific antibody targeting both PD-1 and vascular endothelial growth factor (VEGF), could provide superior progression-free survival (PFS) compared to pembrolizumab in PD-L1-positive NSCLC patients. Given the potential synergy of PD-1 inhibition with anti-angiogenic therapy, ivonescimab represents an innovative approach in the first-line treatment of advanced NSCLC.
Methods and Study Design of Harmoni-2 trial
Patients with locally advanced or metastatic PD-L1-positive NSCLC were randomized to receive ivonescimab or pembrolizumab. The primary endpoint was PFS, while secondary endpoints included overall survival (OS), objective response rate (ORR), safety, and patient-reported outcomes. Adverse events were closely monitored and classified as treatment-related adverse events (TRAEs) and immune-mediated adverse events (irAEs).
This was a randomized, controlled, phase 3 trial conducted in a single-region cohort of Chinese patients with advanced NSCLC. Subgroup analyses were performed based on PD-L1 expression levels and NSCLC histology (squamous vs. non-squamous).
Results of HARMONI-2 trial
Ivonescimab demonstrated superior PFS with a manageable safety profile compared to pembrolizumab, including in squamous NSCLC. The absence of prohibitive toxicity issues suggests its potential as a promising first-line monotherapy for NSCLC. Ongoing trials will further establish its global applicability and role in combination regimens.
-
Efficacy
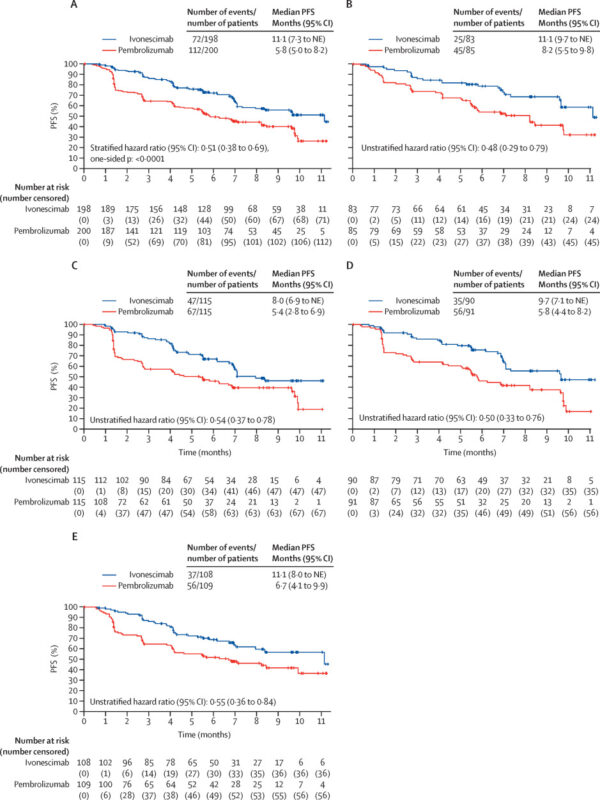
- Ivonescimab demonstrated a statistically significant and clinically meaningful improvement in PFS over pembrolizumab.
- Median PFS: 5.3 months longer with ivonescimab.
- Risk of disease progression or death reduced by 49%.
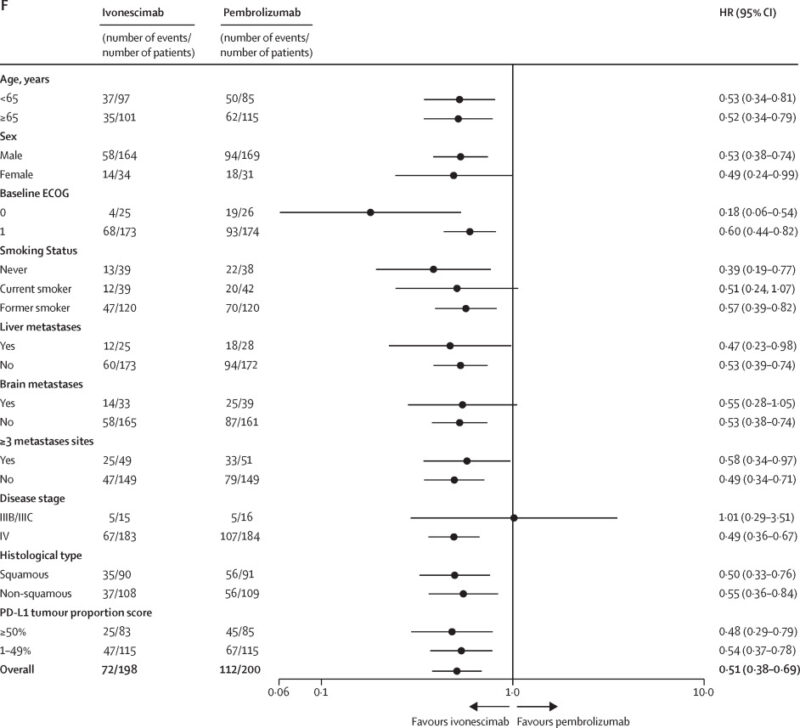
- Efficacy was consistent across PD-L1 expression subgroups (TPS 1-49% and TPS ≥50%) and both squamous and non-squamous NSCLC histologies.
- In squamous NSCLC, ivonescimab significantly improved PFS (HR 0.50; median PFS: 9.7 months).
- Safety
-
-
- TRAEs occurred in 90% of ivonescimab patients vs. 82% in pembrolizumab patients.
- Grade ≥3 TRAEs were more frequent in the ivonescimab group.
- Most common TRAEs: proteinuria, hypertension, laboratory abnormalities (AST increase, hypercholesterolemia, bilirubin increase).
- Serious TRAEs: 21% (ivonescimab) vs. 16% (pembrolizumab).
- Grade ≥3 VEGF-related adverse events were higher in the ivonescimab group (hypertension: 5% vs. 1%, proteinuria: 3% vs. 0%).
- Grade ≥3 hemorrhage was similar in both groups (1% vs. 1%).
- No new safety signals were identified in squamous NSCLC patients, including no increased bleeding risk.
-
Immune-Mediated Adverse Events (irAEs)
- Similar incidence in both groups (ivonescimab: 30%, pembrolizumab: 28%).
- Grade ≥3 irAEs: 7% (ivonescimab) vs. 8% (pembrolizumab).
- Serious irAEs: 6% (ivonescimab) vs. 11% (pembrolizumab).
- No irAEs led to permanent discontinuation of ivonescimab; 5 irAEs led to pembrolizumab discontinuation.
- No irAE-related deaths in either group.
-
-
Patient-Reported Outcomes
- Median time to deterioration in quality of life (EORTC QLQ-C30 global health status) was similar between groups.
- Deterioration-free rate at 12 months: 51% (ivonescimab) vs. 46% (pembrolizumab).
Key Takeaway Messages
- Ivonescimab significantly improves PFS over pembrolizumab in first-line PD-L1-positive NSCLC treatment.
- Efficacy benefits are consistent across histologic subtypes and PD-L1 expression levels.
- Safety profile is manageable, with no new safety concerns or excess bleeding risk in squamous NSCLC.
- Ivonescimab may be a viable chemotherapy-free alternative for PD-L1 TPS 1-49% patients who are ineligible for chemotherapy.
- Further studies, including ongoing global trials (NCT05840016, NCT05899608), will clarify its role in combination with chemotherapy.
You can read the full article here
Written by Sona Karamyan, MD


