Samer Al Hadidi, Associate Professor at UAMS – University of Arkansas for Medical Sciences, shared a post on X:
“An U.S. FDA ODAC will be held May 20th to discuss the potential approval for Daratumumab in smoldering Multiple Myeloma – Smoldering Myeloma.
Very good points made in the discussion.
Few important observations summarised here.”

Anemia as a CRAB criteria occurred in (n=14). It is a number rather than an actual symptom that patient experiences.
In many occasions the initial number is in 11 range.
None of participants received blood transfusions.
None of participants had an AE of fatigue reported.
Note the median and range of Hb in enrolled participants at baseline.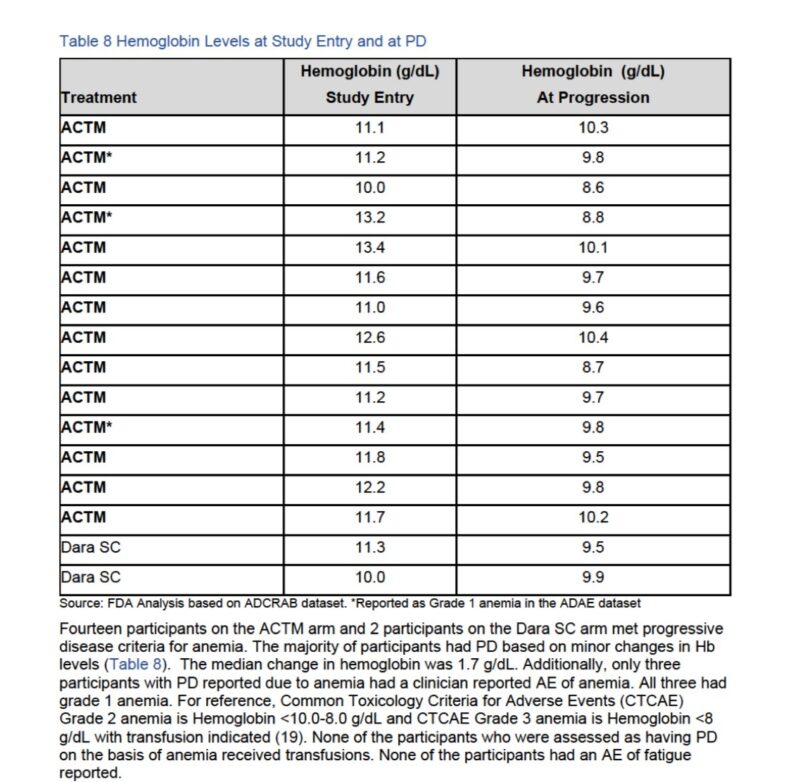
There were more symptomatic bone lesions in the Dara arm compared to the active observation (very interesting).
Of (n=28) in the study reported as bone lesions as part of CRAB, only 3 were symptomatic , 2 of those in the participants who got Dara.

Causes of death are important, note the deaths at 30 days and 60 days.
Most causes were called other, upon request of FDA to get more data on those deaths , details were not available.
This means that early intervention results in better overall survival is not an evidence supported argument.
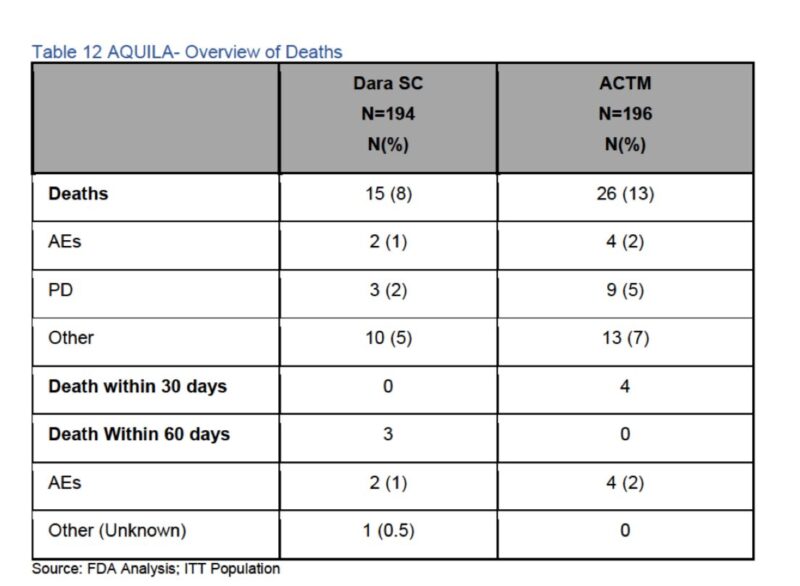
Patient reported outcomes were not appropriately collected and thus considered not informative.
This is important in an asymptomatic condition where we are relying on numbers to offer treatment and we don’t see any difference in serious progression events.

The definition used to call high risk smoldering myeloma was based on criteria not used anymore.
Notice IgA SMM was the risk factor in 25% of pts !!
This is not included in any of the current risk models Most pts enrolled in this study were low or intermediate risk as of most commonly used models nowadays.
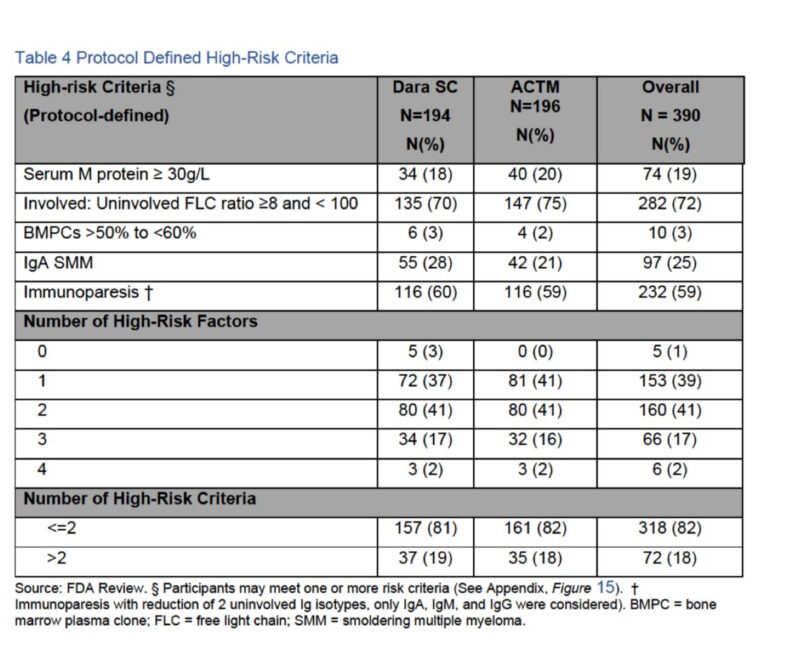
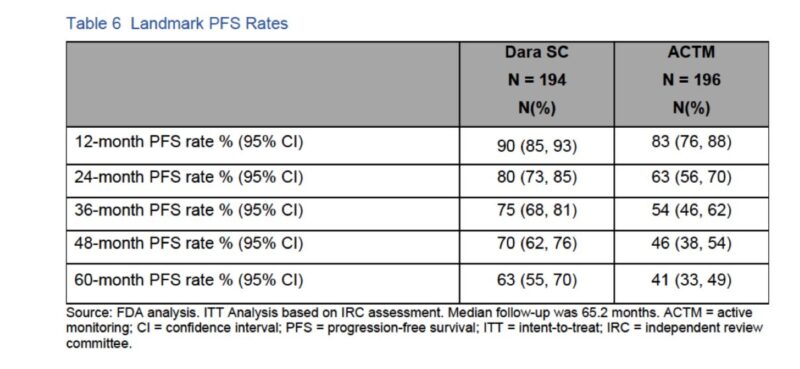
Progression in participants who were observed is quite interesting to think about.
In those considered high risk per study definition , risk of not having progression (which can be a number change) with > 4 years observation is more than 40%.
No much difference in 12-mo PFS rate between both groups with overlapping CI.
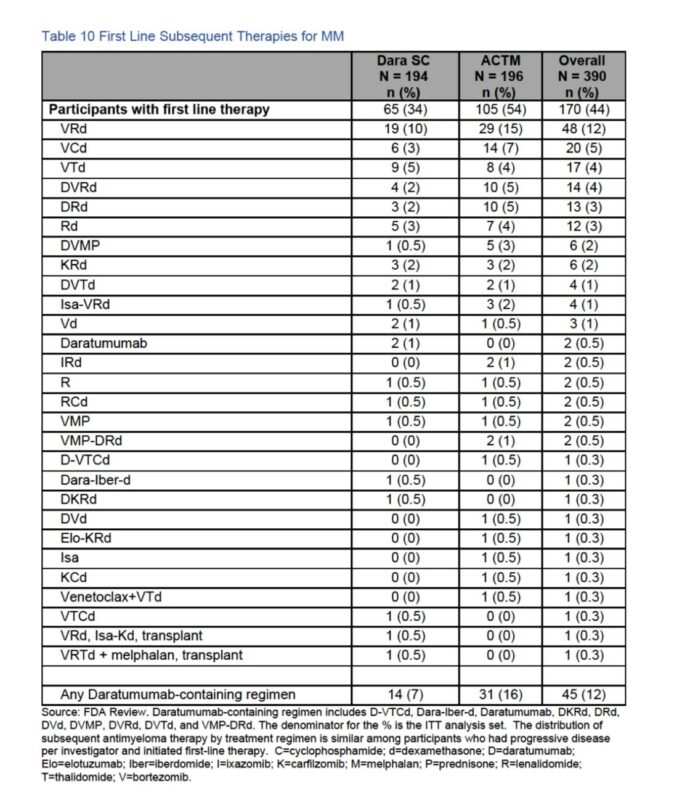
Regiments used when participants needed treatment were really sub-optimal.
Note use of single agent, doublet, outdate triplet and very small number of pts got the today standard of quad based therapy.
I made more points earlier and we summarised in a previous publication.
It will be an interesting discussion but looking at more of those details provided by the briefing , it is becoming clear that there is no clear benefit of early treatment for smoldering myeloma based on what we know.
My recommended ariob will remain to observe all patients regardless of risk.
End.”
More posts featuring Samer Al Hadidi.


