Aakash Desai, Assistant Professor and Associate Director of Phase 1 and Precision Oncology Program at the UAB O’Neal Comprehensive Cancer Center, posted on LinkedIn:
“FDA Accelerated Approval Alert – June 23, 2025.
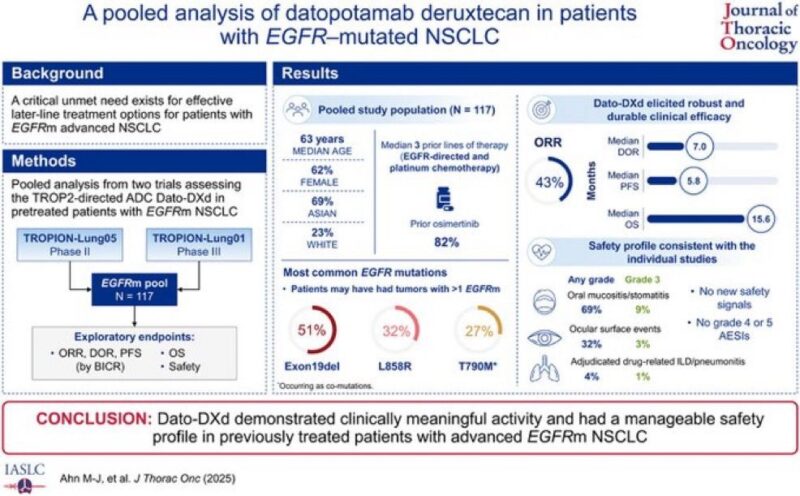
Excited that datopotamab deruxtecan-dlnk (Datroway) has received accelerated approval for patients with EGFR-mutated non-small cell lung cancer (NSCLC) who have progressed on prior EGFR-targeted therapy and platinum-based chemotherapy.
This approval is based on pooled data from:
- TROPION-Lung01 (RCT).
- TROPION-Lung05 (single-arm).
With the following outcomes:
- ORR: 45% (95% CI: 35–54).
- Median Duration of Response: 6.5 months (95% CI: 4.2–8.4).
- AE warnings include ILD/pneumonitis, ocular toxicity, stomatitis.
This adds a critical option in the evolving EGFRm NSCLC treatment landscape.”
More posts featuring Aakash Desai on OncoDaily.


