Roberto Pestana, Clinical Oncologist at Hospital Israelita Albert Einstein, shared on X:
“This week, on May 9-10, the ESMO Virtual Plenary with AACR Expert Commentary will feature a presentation about the duration of adjuvant therapy for patients with localized GIST sarcoma at high risk of relapse. In this thread, I will summarize what we already know about the topic.
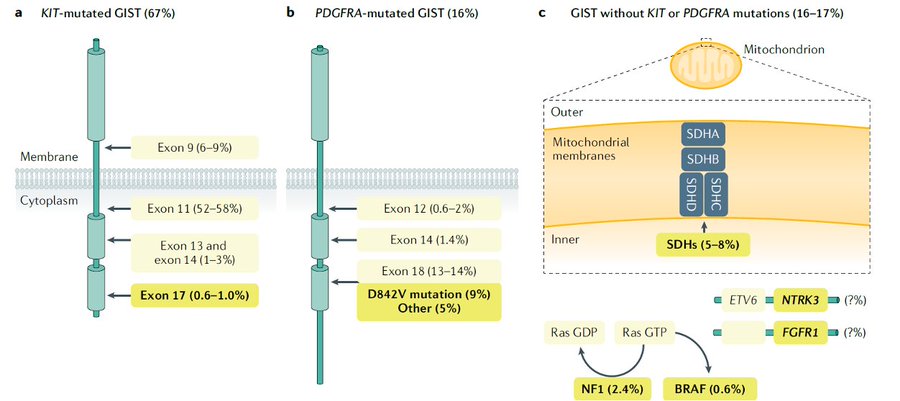
First, GIST is the most common mesenchymal malignancy of the GI tract. It is driven most commonly by KIT mutations, followed by PDGFRA mutations.
There is also a small percentage of so-called wild-type GIST, which is heterogeneous and can be driven by SDH mutations, RAS/RAF mutations, or oncogenic fusions.
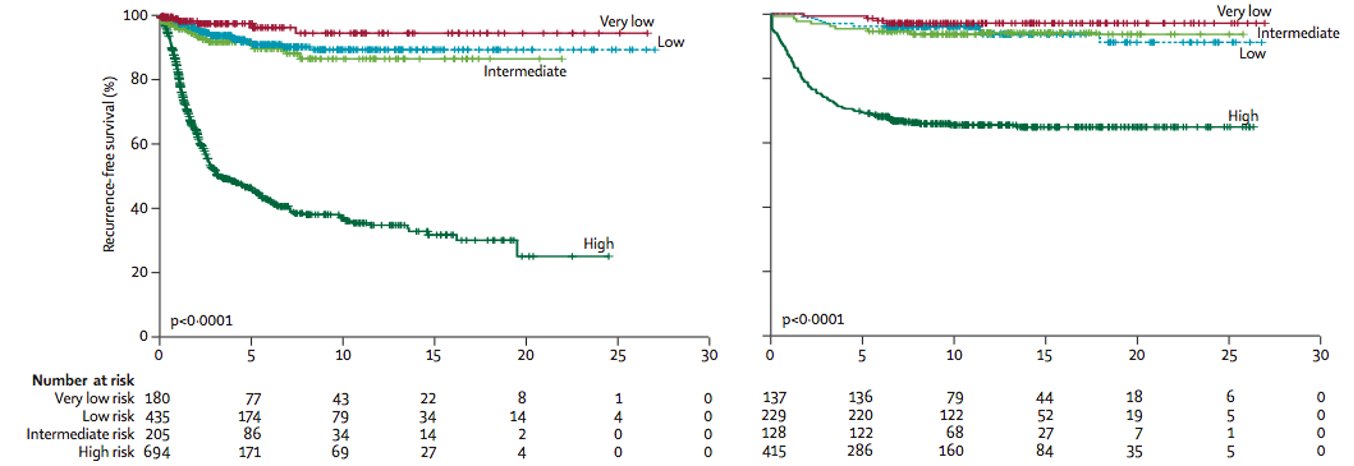
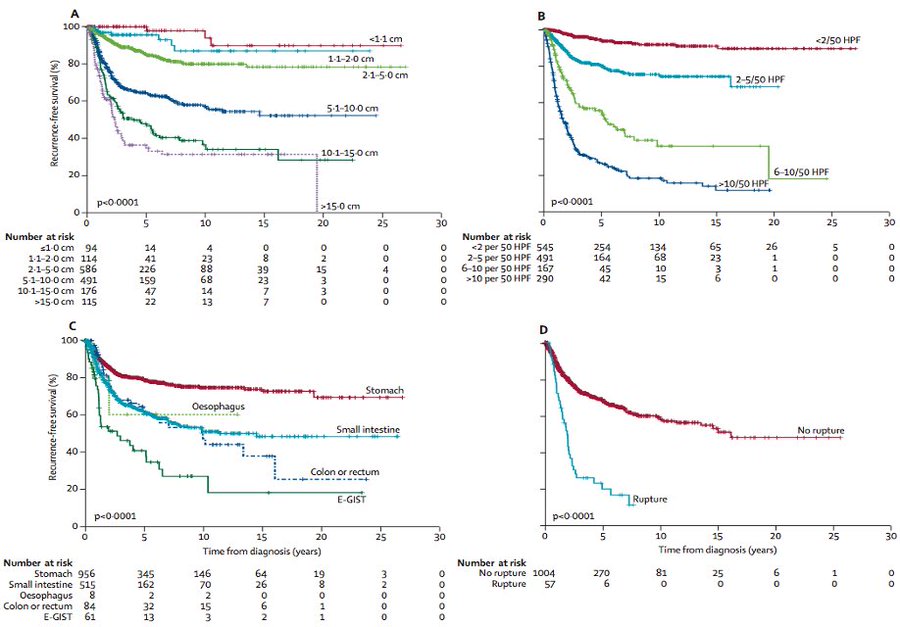
Following resection of localized GIST, the risk of relapse can be determined by location (gastric vs. elsewhere), size, mitotic count, and presence or absence of tumor rupture.
Joensuu. Lancet Oncol 13:265, 2012.
Based on these factors, we can stratify patients in risk groups. Most commonly, this risk is determined by the modified NIH criteria.
Joensuu. Lancet Oncol 13:265, 2012
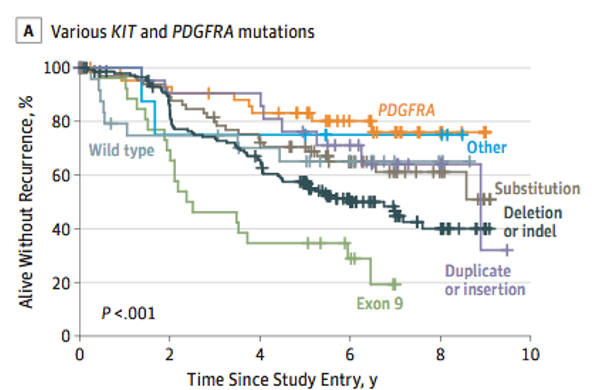
Moreover, genomic is important to guide prognosis and also is predictive of response to therapy.
Joensuu et al. JAMA Oncol, 2017
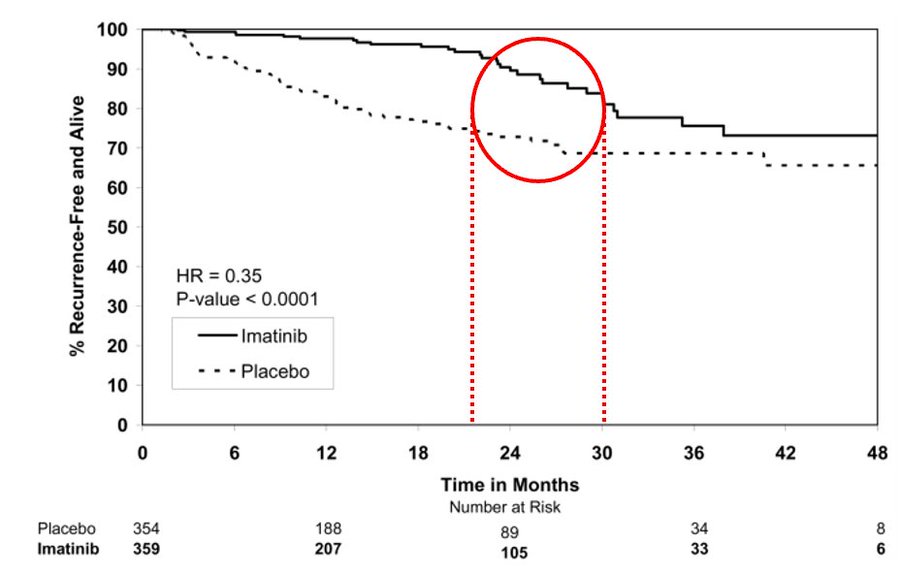
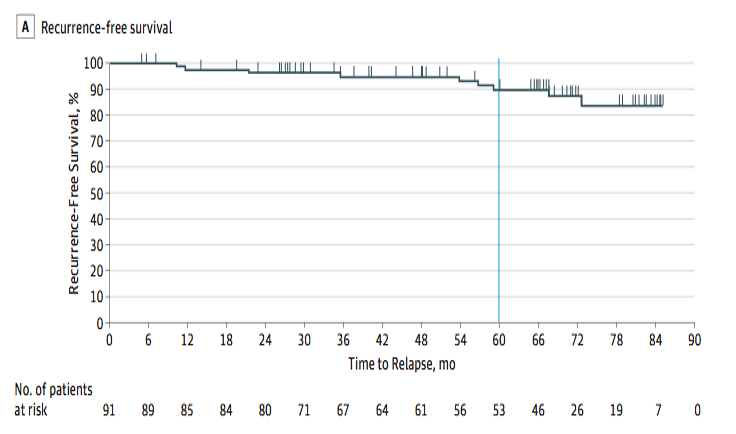
The ACOZOG Z9001 enrolled patients with resected GIST at least 3 cm in size that stained positive for KIT protein. Patients were randomized to 1y of imatinib or placebo.
Imatinib significantly prolonged RFS compared with placebo (98% vs. 83% at 1 year; HR 0.35) However, look at the curves – after 6-18 months of imatinib discontinuation, the imatinib arm curve had a sudden drop. Notably, a heterogeneous population includes low-risk disease.
DeMatteo RP. Lancet 373:1097, 2009.
So….should we extend imatinib? Especially in high-risk disease?
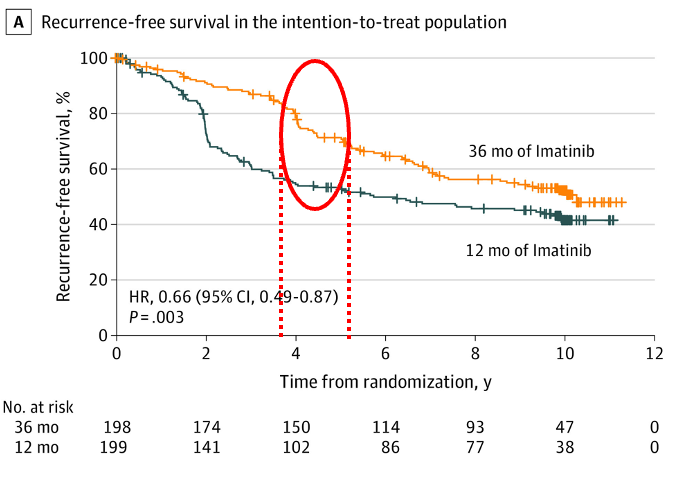
The elegant SSG XVIII trial then randomized patients to 12 months of imatinib (the standard following ACOZOG Z9001) versus 36 months.
This trial, in addition, enrolled a high risk population by NIH criteria. There was a clear PFS benefit of extending therapy – 5y 71% x 53%; 10y 53% x 42%. Of note, there was also an OS benefit, and 36 months of adjuvant imatinib became the standard for high-risk GIST.
However, look at the curves below – even after 36 months of imatinib, when you discontinue the medication, there is a sudden drop in RFS after 6-18 months.
Joensuu H. JAMA Oncol 6:1241, 2020.
Of course, this observation led to many discussions regarding when (if ever) to stop imatinib in adjuvant therapy of high-risk patients.
I would say many of my sarcoma colleagues extend therapy past 3 years in patients with very high-risk disease (such as rupture), especially in patients with sensitive KIT mutations.
In parallel, trials were developed to answer the question of extending treatment. PERSIST-5 is a phase II trial of adjuvant imatinib.
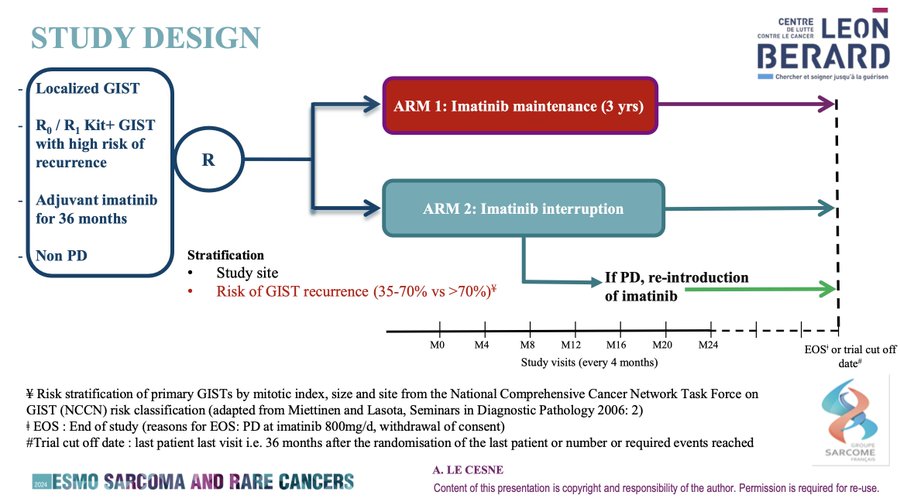
And now we have the firs randomized data to come out, regarding the IMADGIST trial.
This is a multicenter open-label, randomized, phase III study evaluating maintenance of imatinib at the last dose routinely taken by the patient in the 3-year period prior to randomization (either 300 or 400 mg/d) compared to Interruption of imatinib treatment from the day of randomization. Of note, PDGFRA D842V were excluded.
1EP = DFS. 2EPS = OS, among others.
Hopefully, we will soon have a look at longer follow-ups for overall survival and imatinib failure-free survival. Moreover, sub-analyses based on molecular profiling. Looking forward to the presentation! Hopefully, this thread got you all excited about this important data.”
Source: Roberto Pestana/X