Toni Choueiri, the Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute, posted on X about recent paper by him as first author titled “Nivolumab plus relatlimab and nivolumab plus ipilimumab for patients with advanced renal cell carcinoma: results from the open-label, randomised, phase II FRACTION-RCC trial” published on ESMO Open.
Authors: T.K. Choueiri, T.M. Kuzel, S.S. Tykodi, E. Verzoni, H. Kluger, S. Nair, R. Perets, S. George, H. Gurney, R.K. Pachynski, E. Folefac, V. Castonguay, C.-H. Lee, U. Vaishampayan, W.H. Miller Jr., P. Bhagavatheeswaran, Y. Wang, S. Gupta, H. DeSilva, C.-W. Lee, B. Escudier, R.J. Motzer.

“Excited to share the results of FRACTION-RCC, now out in ESMO Open.
FRACTION-RCC is a multicenter, open-label, randomised, phase II trial carried out across several countries. Inclusion criteria were:
– Advanced ccRCC
– > 18 years of age
– KPS ≥70% and life expectancy >3 mo.
Patients enrolled in 1 of 2 tracks based on whether they had received previous anti-PD-1, anti-PD-L1, or anti-CTLA-4 treatment (track 2) or were naive to these therapies (track 1). Patients with progression after treatment in track 1 or 2 were eligible to enrol from track 1 into track 2, or to re-enrol in track 2 to be randomised to receive a new IO treatment combination (NOVEL DESIGN)
Our findings from patients who had received previous anti-PD-1, anti-PD-L1, or anti-CTLA-4 therapy were reported separately.
In track 1 of this study, we focused on the 60 IO-naive pts treated with NIVO + RELA (n = 30) or NIVO + IPI (n = 30) – NIVO + RELA: pts received NIVO 240 mg + RELA 80 mg IV q2w – NIVO + IPI: patients received NIVO 3 mg/kg + IPI 1 mg/kg IV q3w for 4 doses, followed by NIVO 480 mg.
NIVO + RELA group: median follow-up was 48.6 months (IQR 46.9–51.7), ORR 30% (95% CI 15–49%], mDOR 33 weeks (95% CI 16-53), and 24–week PFS rate 43% (95% CI 25–60%). NIVO + IPI group: median follow-up was 48.7 months (IQR 47.1–52.0), ORR 20% (95% CI 8–39%), mDOR not reached (95% CI 33 wks–not estimable), and 24–week PFS rate 49% (95% CI 29–66%).
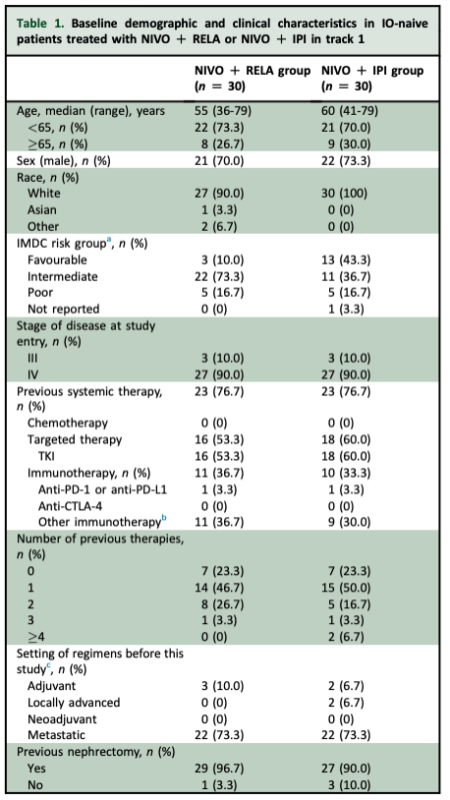
NIVO + RELA group, 25 pts (83%) had a progression event, with mPFS of 4.5 months (95% CI 1.8-7.6 months) and 24-week PFS of 43% (95% CI 25-60%). In the NIVO + IPI group, 18 pts (60%) had a progression event, with mPFS of 3.4 months (95% CI 2.0-23.7 months) and 24-week PFS of 49% (95% CI 29-66%).
NIVO + RELA group: 6/14 pts (43%) with measurable LAG-3 ≥1% vs 1/8 (13%) patients with LAG-3 <1% had an objective response. 5/11 pts (45%) with PD-L1 ≥1% vs 2/11 patients (18%) with PD-L1 <1% had an objective response. NIVO + IPI group: 2/15 (13%) patients with measurable LAG-3 ≥1% vs 4/5 (80%) with LAG-3 <1% had an objective response. 1/5 patients (20%) with assessable PD-L1 ≥1% vs 5/16 patients (31%) with PD-L1 <1% had an objective response.
NIVO + RELA group: mPFS was 6.4 months (95% CI 1.9-10.3) vs 1.6 months (95% CI 0.5-6.2) in pts with assessable LAG-3 expression (≥1% vs <1%) and 6.4 months (95% CI 1.2-13.7) vs 1.8 months (95% CI 1.3-6.2) in patients with assessable PD-L1 expression (≥1% vs <1%). NIVO + IPI group: mPFS was 8.8 months (95% CI 2.0-NE) vs not reached (95% CI 1.4 -NE) in patients with assessable LAG-3 expression (≥1% vs <1%) and 2.6 months (95% CI 1.9 months-NE) vs 9.6 months (95% CI 1.9-NE) in pts with assessable tumor PD-L1 expression (≥1% vs <1%).
NIVO + RELA group: G3+ treatment-related AEs occurred in 4/30 pts (13%). Treatment-related AEs of any grade led to discontinuation in 3 (10%) pts. NIVO + IPI group: G3+ treatment-related AEs occurred in 10/30 (33%). Treatment-related AEs of any grade led to discontinuation in 5 (17%) of 30 pts. No deaths were attributed to study treatment in either group.
In conclusion, NIVO+RELA show antitumor activity and manageable safety in pts with anti-PD-1, anti-PD-L1, or anti-CTLA-4 treatment–naive metastatic ccRCC and our findings support previous studies demonstrating the use of NIVO+IPI as an effective option in patients with mRCC.
Thank you to all the investigators, the study staff, the sponsor Bristol Myers Squibb, and mostly to our patients and their families to whom we devote our work! Fin!”
Toni K. Choueiri is the Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute (DFCI), co-leader of the Kidney Cancer Program at Dana-Farber/Harvard Cancer Center, and the Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School. As a medical oncologist, clinical trialist, and translational researcher, he specializes in treating genitourinary cancers (prostate, bladder, testis, and kidney cancer), with a focus on kidney cancer.


