Vincent Rajkumar, Professor of Medicine at the Mayo Clinic in Rochester and Editor‑in‑Chief at Blood Cancer Journal, shared a post on X:
“Daratumumab is approved in Europe for high-risk smoldering myeloma. This is a big deal for the field and for patients.
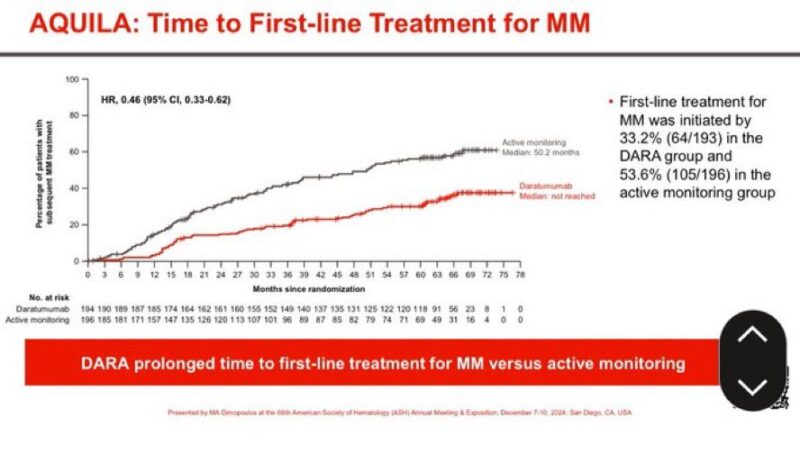
In the AQUILA trial, a finding that I want to highlight is that almost twice as many patients needed to start full therapy for active myeloma with observation than with limited duration (3 years) single-agent daratumumab.
The arm that leads to higher cost and a lot more treatment over the first 6 years (and likely more) is actually the observation arm, not daratumumab.”
Aaron Goodman, Clinical Director at MountainView Hospital and Sarah Cannon Research Institute, shared this post, adding:
“So far this is the best smoldering myeloma study to date.
However, Staring Dara delays time to SLiM myeloma. Is does not prevent irreversible organ toxicity.
These patients can and should be observed. We are over medicalizing society.”
More posts featuring Aaron Goodman and Vincent Rajkumar on OncoDaily.


