The Babak Lab shared a post on LinkedIn:
“COMMIT Trial in dMMR/MSI-H Metastatic Colorectal Cancer
A randomized Phase III study presented at the 2026 ASCO Gastrointestinal Cancers Symposium evaluated the COMMIT regimen, combining atezolizumab (anti–PD-L1) with mFOLFOX6 plus bevacizumab, versus atezolizumab monotherapy in patients with dMMR/MSI-H metastatic colorectal cancer.
Key Focus
The trial tested whether adding chemotherapy and anti-VEGF therapy to immunotherapy can improve outcomes compared with immunotherapy alone in the first-line setting.
Key Results
- Median PFS was 30.0 months with FOLFOX/bev + atezolizumab versus 4.3 months with atezolizumab alone
- ORR was 80.6% vs 46% in favor of the combination
- Disease control rate at 12 months was 62.9% vs 32.4%
- The combination reduced early progression, a known issue with ICI monotherapy in this population
- Toxicity was higher, as expected, and consistent with chemotherapy-based regimens
Conclusion
COMMIT shows that adding chemotherapy and bevacizumab to atezolizumab dramatically improves disease control and durability of response compared with immunotherapy alone in first-line dMMR/MSI-H metastatic colorectal cancer.
Is the ‘triplet regimen’ the new standard for dMMR mCRC despite the added toxicity? Let us know your thoughts below.
Image generated using Sora by OpenAI.”
Maria (Masha) Babak, Head of The Babak Lab and Assistant Professor at City University of Hong Kong, shared this post, adding:
“The median PFS of 4.3 months in the control arm is surprisingly short relative to historical benchmarks. This raises the question: does ‘more’ always equal ‘better’?
The COMMIT trial shows that adding chemo + VEGF to immunotherapy dramatically extends PFS (30 months!), but at the cost of toxicity.
I wonder if this aggressive approach is best reserved for patients facing visceral crisis? For others, the chemo-free route might still be the preferred path.”
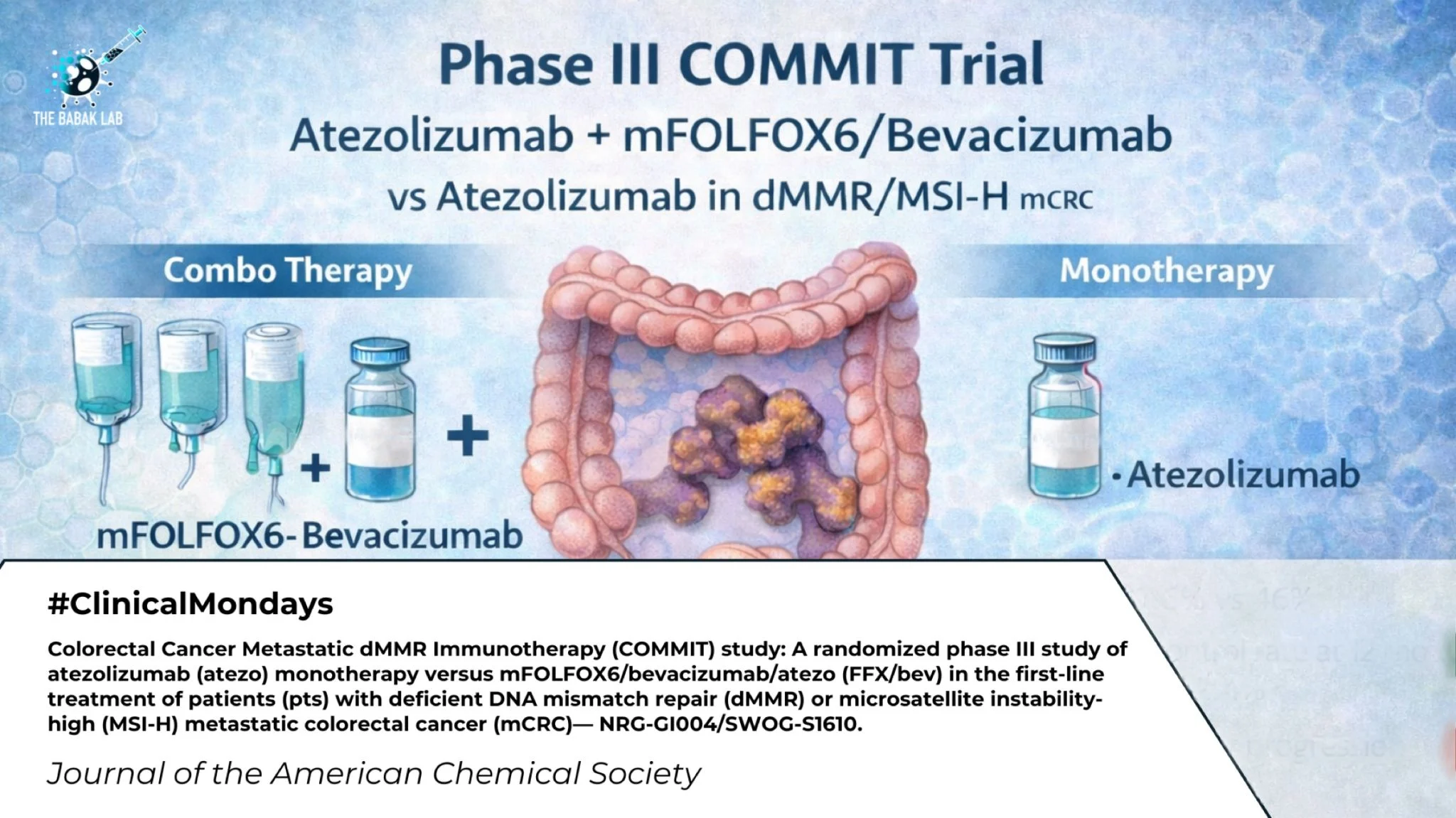
Title: Colorectal Cancer Metastatic dMMR Immunotherapy (COMMIT) study: A randomized phase III study of atezolizumab (atezo) monotherapy versus mFOLFOX6/bevacizumab/atezo (FFX/bev) in the first-line treatment of patients (pts) with deficient DNA mismatch repair (dMMR) or microsatellite instability-high (MSI-H) metastatic colorectal cancer (mCRC)— NRG-GI004/SWOG-S1610.
Authors: Caio Max Sao Pedro Rocha Lima, Greg Yothers, Thomas George, Howard Hochster, Hanna Kelly Sanoff, Deirdre Cohen, Katherine Guthrie, Samuel Jacobs, Anwaar Saeed, Scott Kopetz, Linda Colangelo, Tanner Freeman, Scott Wesley Cole, Dan Sayam Zuckerman, Theodore Hong, Norah Lynn Henry, Patricia Ganz, Charles Blanke, Norman Wolmark, Michael Overman
Read the Full Article in Journal of Clinical Oncology.

More posts featuring The Babak Lab.


