Sergio Cifuentes Canaval, Medical Oncologist at Las Américas Auna Clinic, shared a post on LinkedIn:
“AMALEE Trial: Could a lower starting dose of ribociclib offer safer treatment without compromising efficacy?
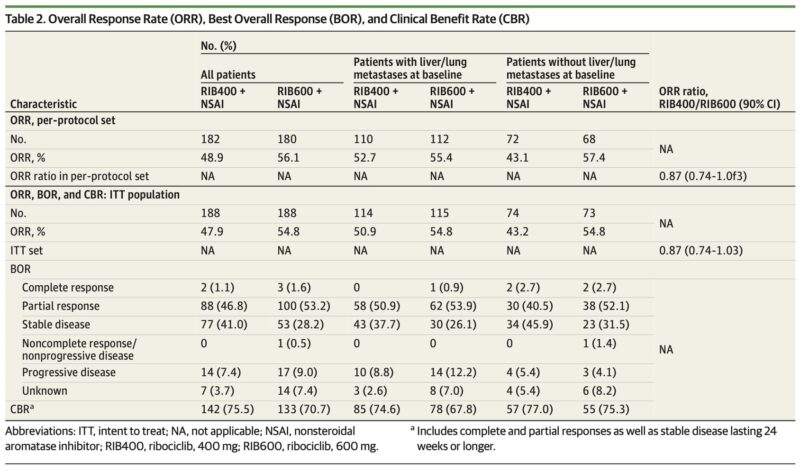
The phase II AMALEE randomized clinical trial compared ribociclib 400 mg vs 600 mg plus a nonsteroidal aromatase inhibitor in patients with HR+/HER2– advanced breast cancer.
Key findings:
Progression-Free Survival (PFS): 26.9 vs 25.1 months (similar)
Duration of Response (DOR): 26.5 vs 28.8 months
Overall Response Rate (ORR): Slightly lower with 400 mg (−7.2%), non-inferiority not met
Safety:
Grade 3–4 neutropenia: 41% vs 58.5%
QTc prolongation: 12.5 ms vs 19.7 ms
Dose reductions: 15.4% vs 36.9%
Although non-inferiority for ORR was not achieved, efficacy outcomes were remarkably similar, while safety significantly improved with the 400 mg dose.
This evidence opens the door for considering lower starting doses — particularly relevant in LMICs settings or in patients with a history of QT prolongation or concomitant QT-prolonging medications, where optimizing safety without losing efficacy is crucial.”
Title: 600- vs 400-mg First-Line Ribociclib in Hormone Receptor–Positive/ERBB2-Negative Advanced Breast Cancer
Journal: JAMA Oncology
Authors: Fatima Cardoso, William Jacot, Sherko Kuemmel, Sudeep Gupta, Felipe Cruz, Rama Balaraman, Ana Ferreira, Tytti Ahola, Yana Chapko, Lyudmila Zhukova, Wendy Chiang, Zheng Li, Yan Ji, Nadia Kaakiou, Natalia Bolotova, and Joseph A. Sparano

More from Sergio Cifuentes Canaval on OncoDaily.


