Rishabh Jain, Medical Oncologist at AIIMS, shared a post on X:
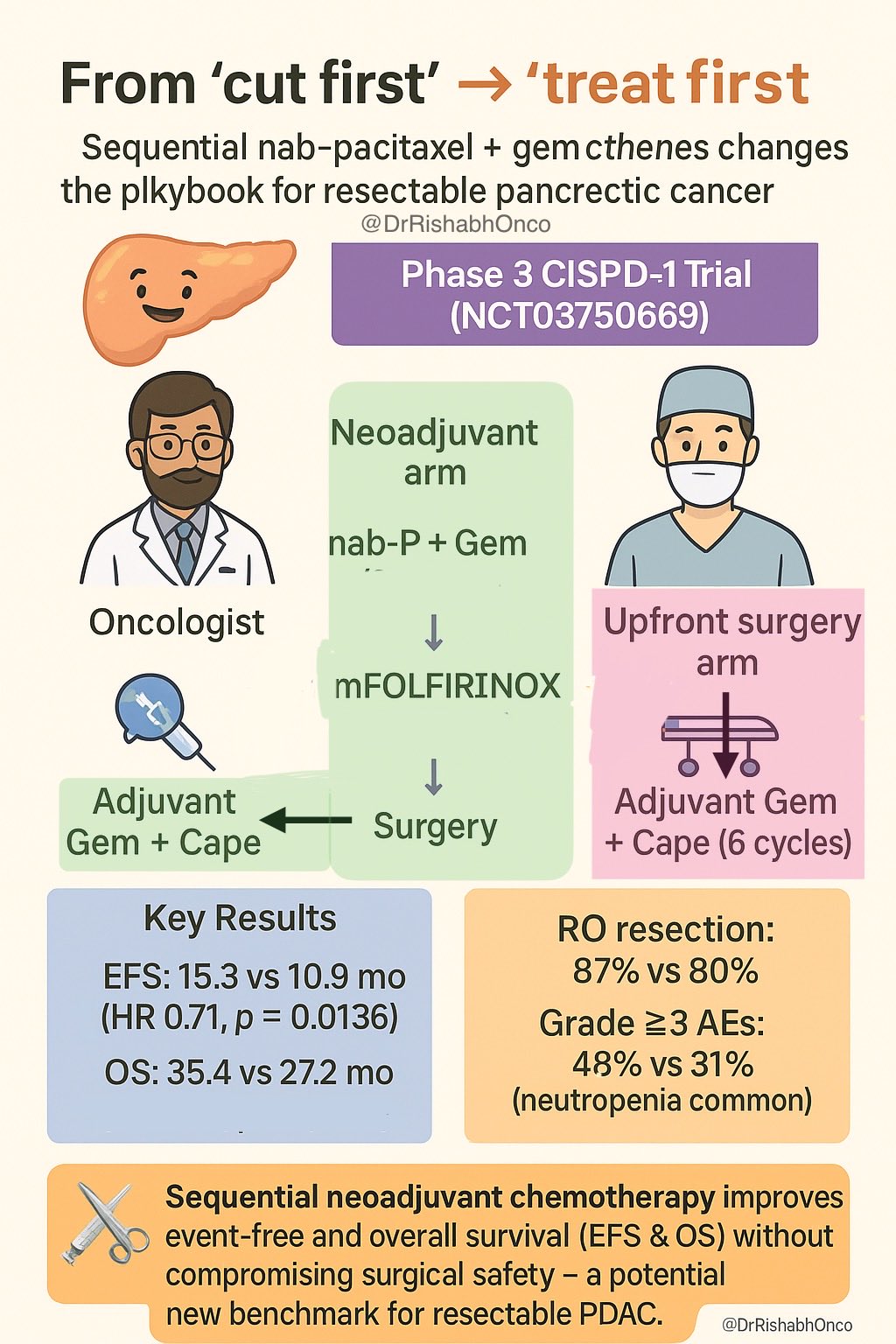
“From “cut first” to “treat first”
Sequential nab-paclitaxel + gemcitabine → mFOLFIRINOX changes the playbook for resectable pancreatic cancer.
- Phase 3 CISPD-1 Trial (NCT03750669)
- 324 pts | Resectable PDAC
Arms
- Neoadjuvant: nab-P + Gem → mFOLFIRINOX → Surgery → 4 cycles Gem + Cape
- Upfront surgery: Surgery → 6 cycles adjuvant Gem + Cape
Results
- EFS 15.3 mo vs 10.9 mo
- HR 0.71 (p = 0.0136)
- OS 35.4 mo vs 27.2 mo
- HR 0.73 (p = 0.0477)
- R0 rate 87 % vs 80 %
- Histopath response 60 %
- Grade ≥3 AEs 48 % vs 31 % (neutropenia common)
- Peri-op mortality ≤ 1 %
Takeaway
Sequential neoadjuvant chemo improves survival without hurting surgical safety -a potential new benchmark for resectable PDAC. Bai et al. Cancer Cell. 2025.”
Title: Neoadjuvant nab-paclitaxel plus gemcitabine followed by modified FOLFIRINOX for resectable pancreatic cancer: A randomized phase 3 trial
Authors: Xueli Bai, Xiang Li, Yiwen Chen, Guoliang Qiao, Qi Zhang, Tao Ma, Shunliang Gao, Min Zhang, Yan Shen, Jian Wu, Jun Yu, Risheng Que, Xiaochen Zhang, Ke Sun, Wenbo Xiao, Tian’an Jiang, Tingbo Liang

More posts featuring Rishabh Jain on OncoDaily.


