Olubukola Ayodele, Breast Cancer Lead at University Hospitals of Leicester NHS Trust, shared a post on LinkedIn:
“ESMO25 | Day 4 Highlights
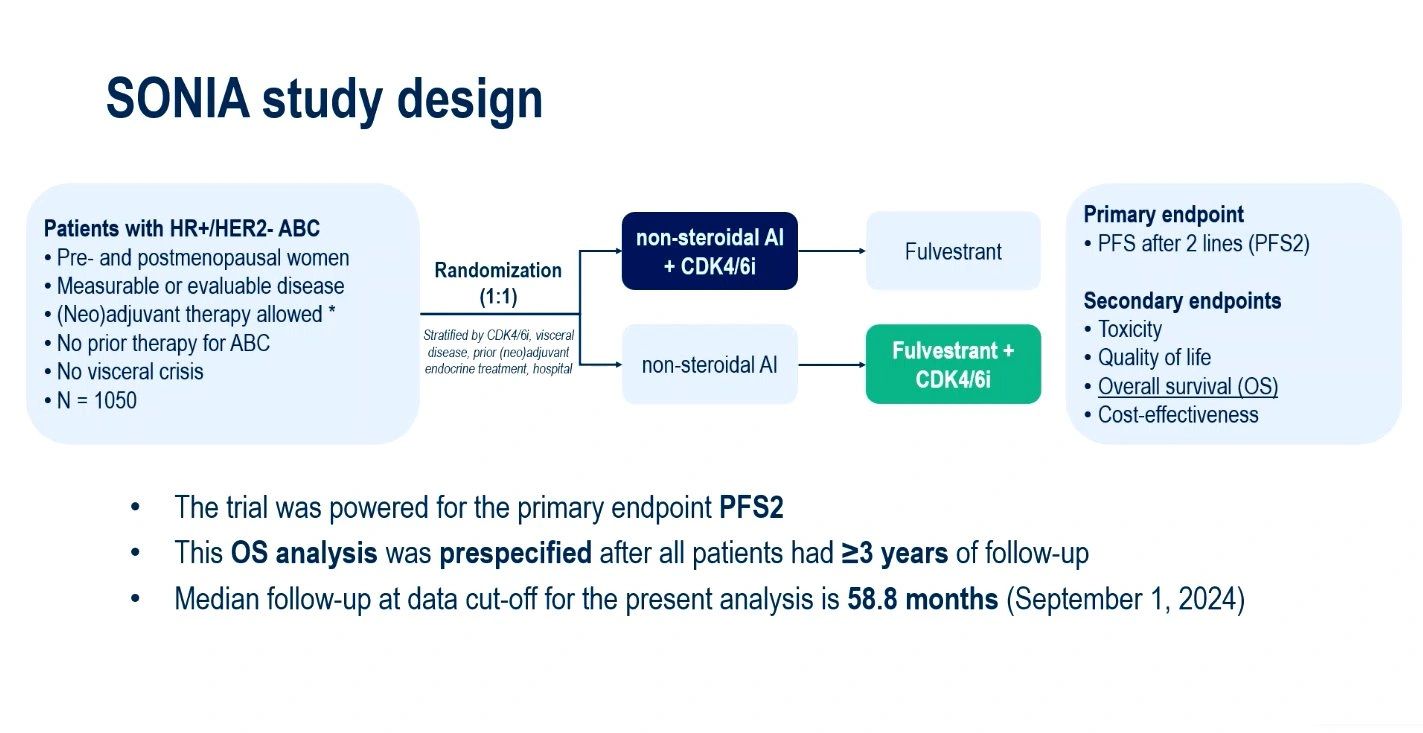
The academic phase III SONIA trial continues to challenge one of the long-standing assumptions in the management of hormone receptor-positive, HER2-negative (HR+/HER2-) advanced breast cancer (ABC): should we always use CDK4/6 inhibitors in the first line?
Trial design
SONIA randomised 1,050 patients with HR+/HER2– ABC to receive:
- Arm 1: Aromatase inhibitor (AI) + CDK4/6 inhibitor → fulvestrant on progression
- Arm 2: AI – fulvestrant + CDK4/6 inhibitor on progression
The primary endpoint was progression-free survival (PFS) after two lines of therapy, which showed no significant benefit for first-line CDK4/6 inhibitor use (HR 0.87; 95% CI 0.74–1.03).
This year at ESMO 2025, we saw the prespecified overall survival (OS) analysis.
Key results
After a median follow-up of 58.5 months, 606 deaths had occurred:
- Median OS: 47.9 months (CDK4/6 first) vs 48.1 months (CDK4/6 second)
- HR 0.91; 95% CI 0.77–1.07; p=0.24
In other words, no statistically or clinically meaningful OS difference between first-line and second-line CDK4/6 inhibitor use. Importantly, toxicity and cost were higher when used first-line, without additional benefit.
Results were consistent across subgroups and CDK4/6 inhibitor type (palbociclib or ribociclib).
Caveats/caution
A post-hoc analysis revealed a notable signal: In premenopausal patients, there was a trend toward OS benefit with first-line use (HR 0.53; 95% CI 0.27–1.02).
No such benefit was seen in postmenopausal women (HR 1.00; 95% CI 0.80–1.25; p = 0.01).
Low OS compared to historical trials and non-standard of care of single agents (however, we still see some trials using single agent fulvestrant as control, which shouldn’t be encouraged). Given the expanding landscape of molecularly guided therapies, deferring CDK4/6 inhibitors to later lines seems less likely in real-world practice.
Why it matters
This finding aligns with what many oncologists observe in practice; younger/ premenopausal women often have more aggressive biology, and may indeed benefit from early intensification with CDK4/6 inhibitors.
However, for many postmenopausal patients, these data suggest that deferring CDK4/6 inhibitors to second line could be a rational, cost-effective, and less toxic strategy, without compromising survival.
Pragmatic implications
In health systems where cost-effectiveness and value-based care are priorities, SONIA provides evidence to individualise therapy rather than defaulting to universal first-line CDK4/6 use.
Future research would need to focus on identifying biological or genomic markers that distinguish those who don’t truly benefit from early intensification.
This study reinforces the importance of nuanced decision-making: the best first-line treatment may not be the same for everyone.”
Follow the latest ESMO 2025 news on OncoDaily.


