Olivier Elemento, Director of Englander Institute for Precision Medicine at Weill Cornell Medicine, shared a post on LinkedIn:
“From Algorithm to Bedside: Solving a Critical Surgical Challenge
Bringing an artificial intelligence model from a research paper into a real-world clinical workflow is incredibly challenging. Many promising algorithms never make the leap from publication to patient care.
A new study in Nature Medicine by Andreas Weinberger Rosen, Ilze Ose, Ismail Gogenur, and colleagues offers a powerful roadmap for navigating these challenges by tackling a critical clinical problem.
The Clinical Problem: Predicting Surgical Risk
After major cancer surgery, some patients recover smoothly while others suffer from severe complications that can impact survival and increase healthcare costs. A core challenge in modern surgery is accurately identifying these high-risk patients beforehand. Risk is multifactorial, depending on a complex mix of age, comorbidities, and cancer stage, making it difficult for single-dimensional tools to capture the full picture.
Building the Model
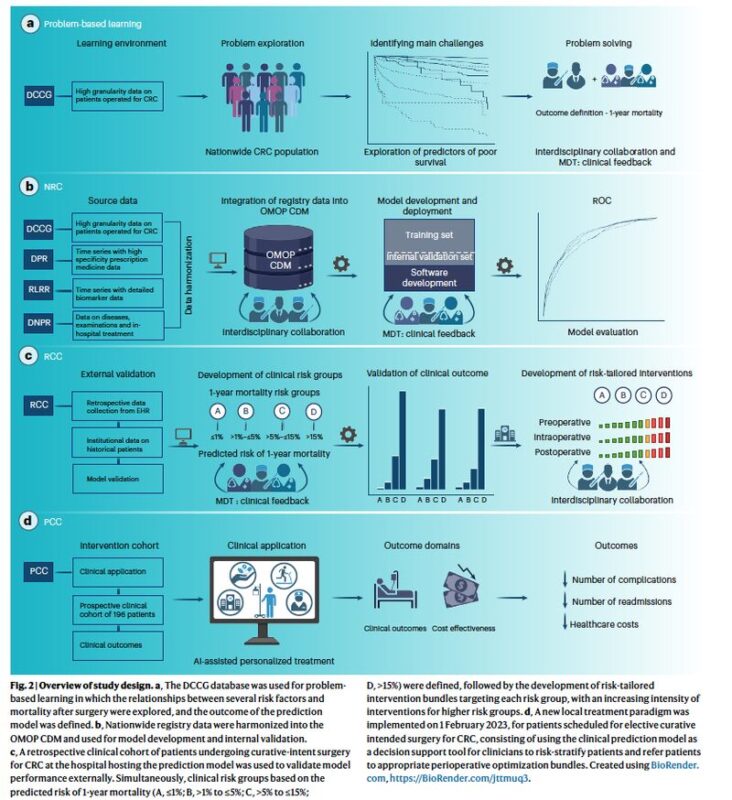
The team developed their model using data from four Danish national registries, encompassing over 18,400 patients undergoing colorectal cancer surgery. The result is a LASSO logistic regression model – a great example, I think, that useful predictive models do not always need to be complex deep learning systems. It uses 58 variables, including patient demographics, lab values, comorbidities, and clinical scores, to make its prediction. The model achieved strong performance with an Area Under the Receiver Operating Characteristic (AUROC) of 0.79 on external validation data.
From Prediction to Improved Outcomes
A key to its clinical utility is that the model’s output was turned into actionable risk groups (A, B, C, or D) that are registered in the patient’s EHR to guide personalized perioperative care. Each risk group is linked to a standardized bundle of interventions, with the intensity and frequency of care escalating with the predicted risk. For instance, patients in the highest-risk group (D) were automatically admitted to the postoperative care unit for 24 hours for closer monitoring. This strategy led to a significant drop in severe complications (from 28.0% to 19.1%) and proved cost-effective, saving over $2,800 per patient.
Building on This Success
The authors are transparent that this was a nonrandomized before/after study, which provides powerful real-world evidence but cannot definitively prove a causal link. Looking forward, full integration of the data workflow into the EHR will be key to facilitating broader adoption, eventually replacing the current need for surgeons to manually extract data points.
Despite these limitations, this study is a powerful proof-of-concept of clinical AI deployment, providing a transparent and effective framework for using AI to solve a critical clinical problem.”
Title: Clinical implementation of an AI-based prediction model for decision support for patients undergoing colorectal cancer surgery
Authors: Andreas Weinberger Rosen, Ilze Ose, Mikail Gögenur, Lars Peter Kloster Andersen, Rasmus Dahlin Bojesen, Rasmus Peuliche Vogelsang, Martin Høyer Rose, Philip Wallentin Steenfos, Lasse Bremholm Hansen, Helle Skadborg Spuur, Ines Raben, Søren Thorgaard Skou, Ellen Astrid Holm, Karina Mortensen, Trine Kjær, Jens Ravn Eriksen, Ismail Gögenur
Read the Full Article in Nature Medicine.

Title: Simple Linear Cancer Risk Prediction Models With Novel Features Outperform Complex Approaches
Authors: Scott Kulm, Lior Kofman, Jason Mezey, Olivier Elemento
Read the Full Article in JCO Clinical Cancer Informatics.

More posts featuring Olivier Elemento on OncoDaily.


