Marco Donia, Research Group Leader of the TIL group at CCIT (Center for Cancer Immune Therapy) and Professor at the University of Copenhagen, shared a post on LinkedIn:
“Neoadjuvant intralesional immunocytokines for macroscopic, resectable stage ≥III melanoma
Title: Final 5-Year Follow-Up Results Evaluating Neoadjuvant Talimogene Laherparepvec Plus Surgery in Advanced Melanoma
Authors: Reinhard Dummer, David E. Gyorki, John R. Hyngstrom, Meng Ning, Tatiana Lawrence, Merrick I. Ross
Read the full Article on Jama Network.

Neo-adjuvant immunotherapy with checkpoint inhibitors is a new standard-of-care for patients with macroscopic, resectable stage ≥III melanoma (Read further). Is there a role for intralesional therapies?
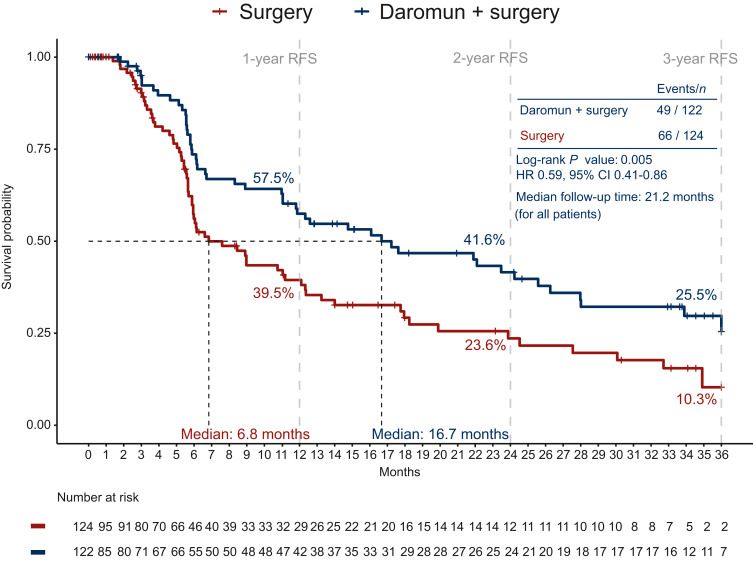
Phase III PIVOTAL trial: intralesional daromun (L19IL2 + L19TNF) weekly for 4 weeks before surgery vs. upfront surgery in resectable stage ≥III melanoma
Key points
- 246 patients enrolled, most heavily pretreated (74% ≥2 prior surgeries; 35% prior systemic therapy – meaning including a patient group with recurrence after prior anti-PD-1).
- Recurrence-free survival (RFS): median 16.7 months with daromun vs. 6.8 months with surgery alone (HR 0.59; P=0.005);
- Distant-metastasis free survival (DMFS): median 28 months with daromun vs 17.7 with surgery alone (HR 0.60, P = 0.029).
- Safety: 26.3% Treatment-related grade ≥3 adverse events (11% were injection-site reactions)
Take-home messages
- Neoadjuvant daromun is safe and has activity (RFS and DMFS improvement), even in a high-risk, pretreated population.
Clinical implications
- Immune checkpoint inhibitors remain the current standard of care
- Is there a potential space for Daromun/intralesional therapies in the neo-adjuvant space? Perhaps for patients with locoregional recurrence after prior anti-PD-1 therapy, or not eligible for checkpoint inhibitors (however, the study did not formally test this)
while criteria for determining a ‘recurrence event’ are different in neo-adjuvant trials and only ~1/3 of the patients in this study received adjuvant therapy, the 1-year RFS in the control group of PIVOTAL had a worse RFS (39%) compared to control groups of trials of adjuvant and neo-adjuvant therapy , potentially reflecting a population with worse baseline features.
Congratulations to the authors for this important study!”
Title: Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma
Authors: Christian U. Blank, Minke W. Lucas, Richard A. Scolyer, Bart A. van de Wiel, Alexander M. Menzies, Marta Lopez-Yurda, Lotte L. Hoeijmakers, Robyn P.M. Saw, Judith M. Lijnsvelt, Nigel G. Maher, Saskia M. Pulleman, Maria Gonzalez, Alejandro Torres Acosta, Winan J. van Houdt, Serigne N. Lo, Anke M.J. Kuijpers, Andrew Spillane, W. Martin C. Klop, Thomas E. Pennington, Charlotte L. Zuur, Kerwin F. Shannon, Beatrijs A. Seinstra, Robert V. Rawson, John B.A.G. Haanen, Sydney Ch’ng, Kishan A.T. Naipal, Jonathan Stretch, Johannes V. van Thienen, Michael A. Rtshiladze, Sofie Wilgenhof, Rony Kapoor, Aafke Meerveld-Eggink, Lindsay G. Grijpink-Ongering, Alexander C.J. van Akkooi, Irene L.M. Reijers, David E. Gyorki, Dirk J. Grünhagen, Frank M. Speetjens, Sonja B. Vliek, Joanna Placzke, Lavinia Spain, Robert C. Stassen, Mona Amini-Adle, Céleste Lebbé, Mark B. Faries, Caroline Robert, Paolo A. Ascierto, Rozemarijn van Rijn, Franchette W.P.J. van den Berkmortel, Djura Piersma, Andre van der Westhuizen, Gerard Vreugdenhil, Maureen J.B. Aarts, Marion A.M. Stevense-den Boer, Victoria Atkinson, Muhammad Khattak, Miles C. Andrews, Alfons J.M. van den Eertwegh, Marye J. Boers-Sonderen, Geke A.P. Hospers, Matteo S. Carlino, Jan-Willem B. de Groot, Ellen Kapiteijn, Karijn P.M. Suijkerbuijk, Piotr Rutkowski, Shahneen Sandhu, Astrid A.M. van der Veldt, Georgina V. Long
Read The Full Article on The new England journal of Medicine

Title: Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma
Authors: Christian U. Blank, Minke W. Lucas, Richard A. Scolyer, Bart A. van de Wiel, Alexander M. Menzies, Marta Lopez-Yurda, Lotte L. Hoeijmakers, Robyn P.M. Saw, Judith M. Lijnsvelt, Nigel G. Maher, Saskia M. Pulleman, Maria Gonzalez, Alejandro Torres Acosta, Winan J. van Houdt, Serigne N. Lo, Anke M.J. Kuijpers, Andrew Spillane, W. Martin C. Klop, Thomas E. Pennington, Charlotte L. Zuur, Kerwin F. Shannon, Beatrijs A. Seinstra, Robert V. Rawson, John B.A.G. Haanen, Sydney Ch’ng, Kishan A.T. Naipal, Jonathan Stretch, Johannes V. van Thienen, Michael A. Rtshiladze, Sofie Wilgenhof, Rony Kapoor, Aafke Meerveld-Eggink, Lindsay G. Grijpink-Ongering, Alexander C.J. van Akkooi, Irene L.M. Reijers, David E. Gyorki, Dirk J. Grünhagen, Frank M. Speetjens, Sonja B. Vliek, Joanna Placzke, Lavinia Spain, Robert C. Stassen, Mona Amini-Adle, Céleste Lebbé, Mark B. Faries, Caroline Robert, Paolo A. Ascierto, Rozemarijn van Rijn, Franchette W.P.J. van den Berkmortel, Djura Piersma, Andre van der Westhuizen, Gerard Vreugdenhil, Maureen J.B. Aarts, Marion A.M. Stevense-den Boer, Victoria Atkinson, Muhammad Khattak, Miles C. Andrews, Alfons J.M. van den Eertwegh, Marye J. Boers-Sonderen, Geke A.P. Hospers, Matteo S. Carlino, Jan-Willem B. de Groot, Ellen Kapiteijn, Karijn P.M. Suijkerbuijk, Piotr Rutkowski, Shahneen Sandhu, Astrid A.M. van der Veldt, Georgina V. Long
Read The Full Article on The new England journal of Medicine
More from Marco Donia on OncoDaily.


