Julian Chavarriaga, Urologic Oncologist at Fundacion CTIC, shared a post on X:
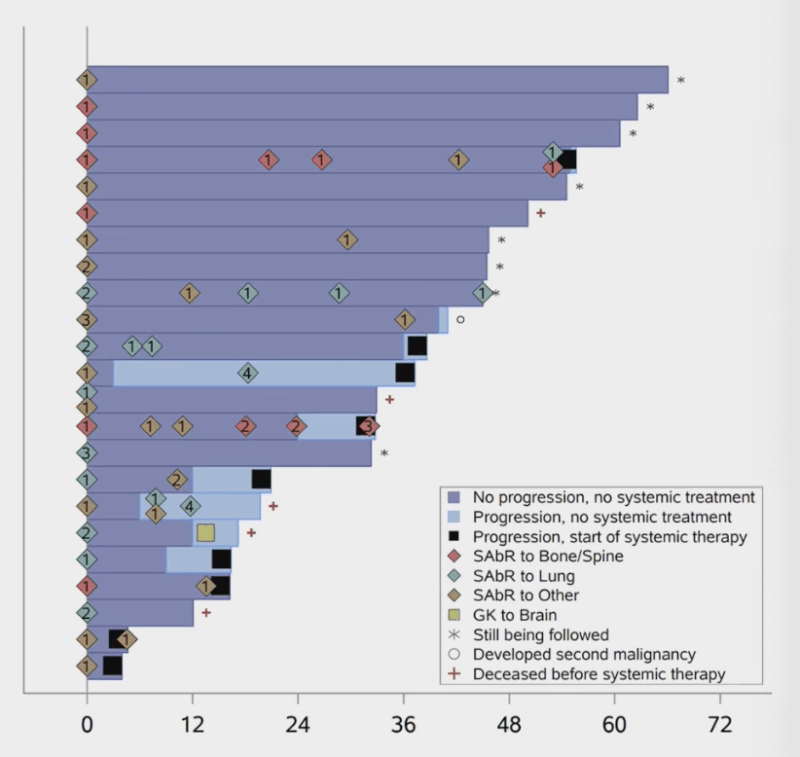
“ASTRO25 Phase IIb trial of 1L SAbR in oligometastatic RCC (≤3 mets, no systemic Rx)
Purpose: Delay systemic therapy, reduce toxicity
23 pts | 69 lesions | IMDC favorable/intermediate
100% LC / 1-year systemic therapy-free: 91%
Median TTST: 55.6 mo
mPFS: 40 mo | OS 3y: 68.7% | CSS 3y: 87.0% PFS on systemic Rx: 5.7 mo (post-SAbR)
QoL: Stable over time
Supports upfront SAbR in select RCC but criteria must be refined / Phase III needed”
You can also read:



