The 63rd Annual Meeting of the Japan Society of Clinical Oncology (JSCO 2025) was held from October 16 to 18, 2025, at Pacifico Yokohama in Japan. The meeting’s theme, “Living with Cancer, Living through Cancer,” reflected a growing focus on not only improving survival through advanced therapies but also supporting patients as they navigate life during and after cancer treatment.
JSCO 2025 brought together oncologists, researchers, and healthcare professionals from around the world to discuss the latest innovations in cancer care, multidisciplinary collaboration, and the integration of new technologies such as AI and robotics into clinical oncology.
Estela Rodriguez, Associate Director of Community Outreach and Co-Lead of Thoracic Site Disease Group of the Sylvester Comprehensive Cancer Center, shared some key highlights from the Japan Society of Clinical Oncology Meeting:
“Honored to be invited to Yokohama, Japan this week for the JSCO25, Japanese Society of Clinical Oncology meeting to Co-chair the ASCO/ JSCO Fellowship Program session.”

“Great to catch up with IASLC board member Hidehito HORINOUCHI in Japan during JSCO25/ ASCO Joint Symposium.
Being part of this global community has been one of the most rewarding parts of my career.”

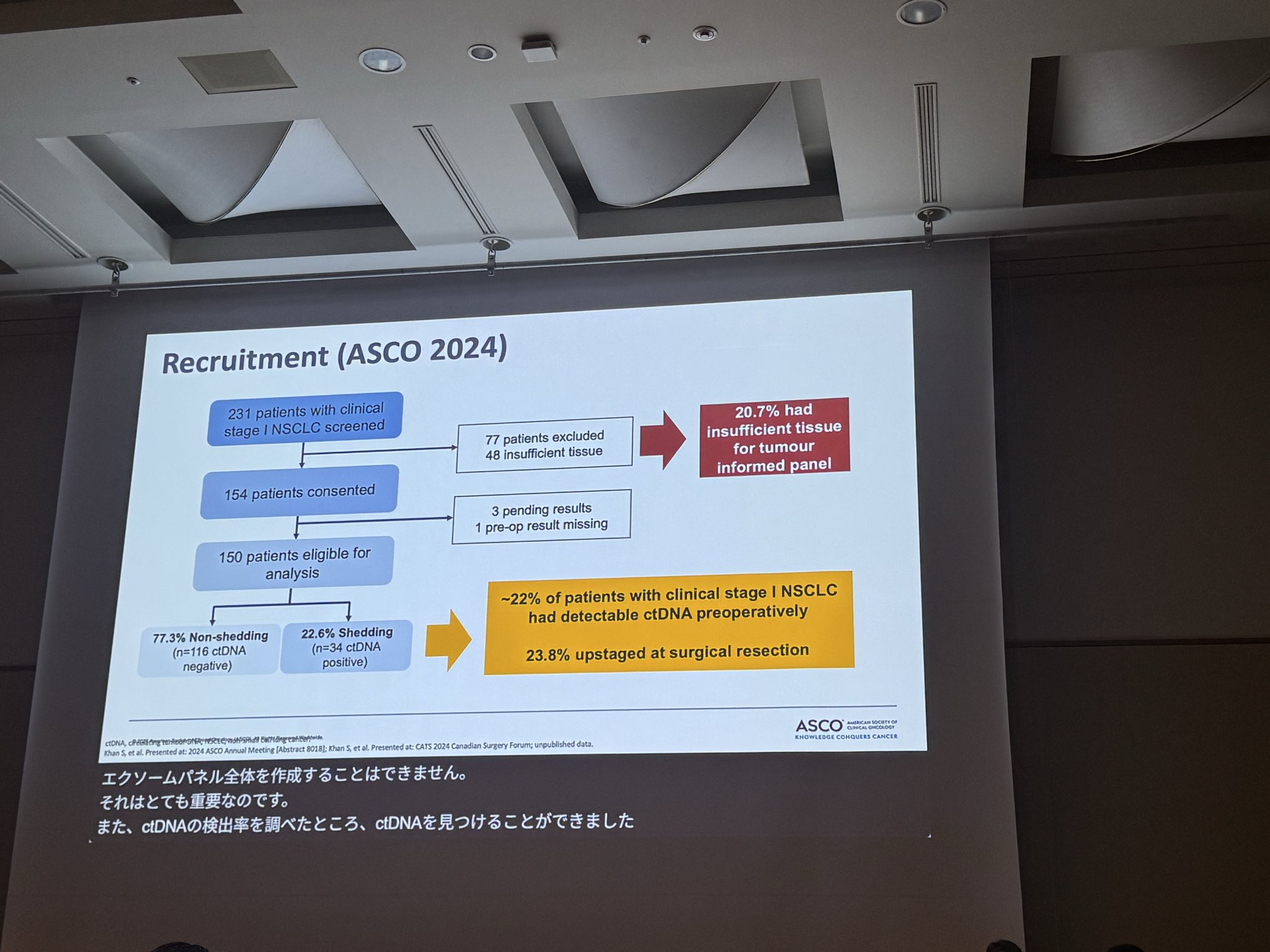
“JSCO25 -ASCO Joint Symposium: Dr Natasha Leighl gives an excellent presentation on clinical utility of ctDNA in early stage lung cancer- so far not ready for prime time due to issues with:
- false negatives/test sensitivity
- standardization of results
- clinical utility
- added MRD testing anxiety for patients.”
“Great reconnecting at JSCO25 with my ASCO-JSCO fellowship mentee, Dr. Yohei Asano, orthopedic oncologist from Kanazawa University Hospital.
Wonderful cultural and academic exchange on the management of bone metastases during his visit to Sylvester Comprehensive Cancer Center.”

“JSCO25 – ASCOJoint Symposium: Benjamin Garmezy presents on ctDNA utility in perioperative GU cancer for escalation/ escalation of adjuvant immunotherapy (bladder> renal) where there are multiple trials w tumor-informed panels that have shown clinical utility in prospective trials.”

More posts featuring Estela Rodriguez.


