Harpreet Singh, Chief Medical Officer at Precision for Medicine, shared a post on LinkedIn:
“Small cell lung cancer (SCLC) was a critical topic at World Lung 2025, and rightly so. SCLC continues to present one of the greatest unmet needs in oncology. What stood out this year was not just the volume of discussion, but the diversity of modalities, from T-cell engagers to radiopharmaceuticals and ADCs, each signaling that the treatment landscape may finally be shifting.
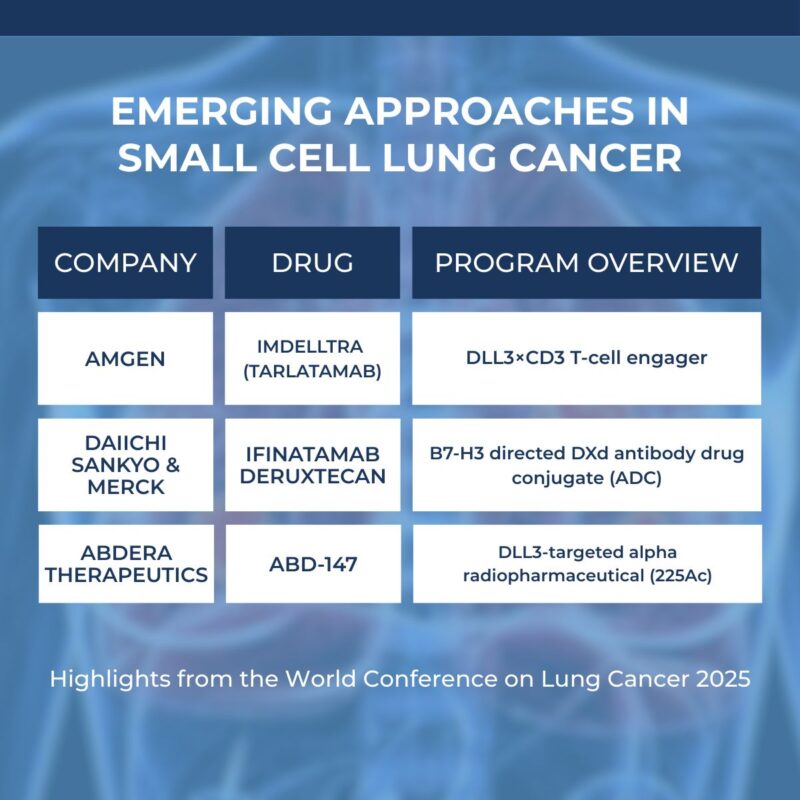
Three programs stood out:
- Imdelltra (tarlatamab from Amgen) a DLL3×CD3 T-cell engager for relapsed SCLC was granted accelerated approval last year. Phase 3 DeLLphi-304 showed median OS 13.6 vs 8.3 months versus chemo with improved tolerability. First-line studies (DeLLphi-305, -306, -312) are ongoing. Amgen
- ABD-147 (Abdera) a DLL3-targeted alpha radiopharmaceutical using Actinium-225 with Fast Track and Orphan Drug designations. Demonstrated a very promising early phase 1a trial that showed tumor-selective uptake and disease stabilization, with no dose-limiting toxicities. Dose escalation continues. Abdera Therapeutics
- Ifinatamab deruxtecan (I-DXd from Daiichi Sankyo/Merck) a B7-H3 ADC with FDA Breakthrough Therapy Designation granted for SCLC patients who have progressed on or after chemo (Aug 2025). Phase 2 IDeate-Lung01 demonstrated Overall ORR 48.2% (95% CI: 39.6–56.9); second-line ORR 56.3% (95% CI: 37.7–73.6). Their presentation included an exploratory analysis of 65 patients with brain metastases at baseline, recording an intracranial ORR 46.2% (95% CI: 33.7–59.0). Phase 3 OS-focused trial is underway.
Collectively, these data suggest that mechanistic innovation, whether through DLL3 or B7-H3 targeting, can open new paths forward for patients who have historically had few options. As these programs evolve, careful attention to study design, safety, and durability will be critical.”
More posts featuring World Lung 2025.


