Drew Moghanaki, Professor, Chief of Thoracic Oncology at the Department of Radiation Oncology and Stanley Iezman and Nancy Stark Endowed Chair in Thoracic Radiation Oncology Research at David Geffen School of Medicine at UCLA, and Chief Medical Officer of Respirati, shared a post on X:
“Time for an X-torial about key findings from the EA5181 study reported at WCLC25, which was a phase III randomized trial investigating different drug combinations with chemoradiotherapy for stage III NSCLC.
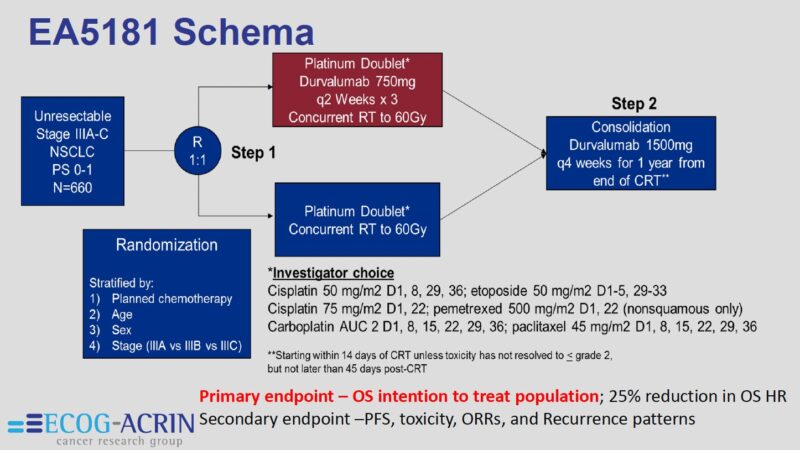
The study enrolled a mix of stage IIIA, IIIB, and IIIC patients who are generally not candidates for surgery. As such, they are pre-determined to have inferior outcomes compared to operable patients enrolled in studies investigating novel drugs + surgery for stage III NSCLC.
The results didn’t show any differences in safety or efficacy between study arms, confirming the futility of adding immunotherapy during CRT.
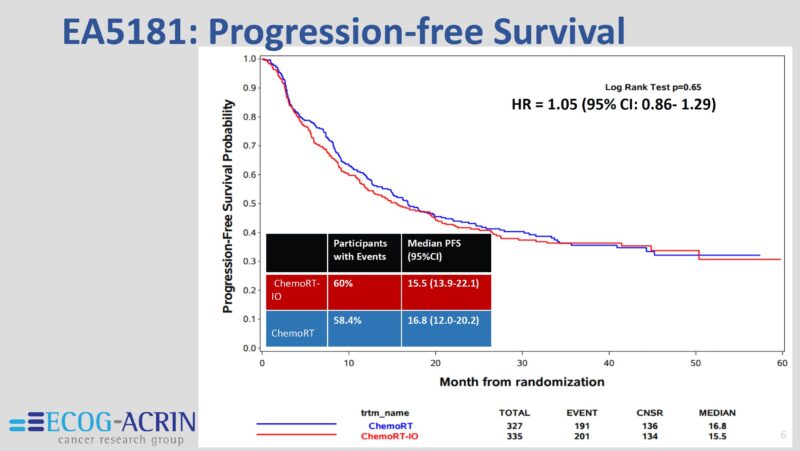
What stands out to me from the results is the extraordinary PFS of 40% at 3 years in the unselected cohort, which I don’t think can be achieved with drugs and surgery for this population, where half = IIIB + IIIC.
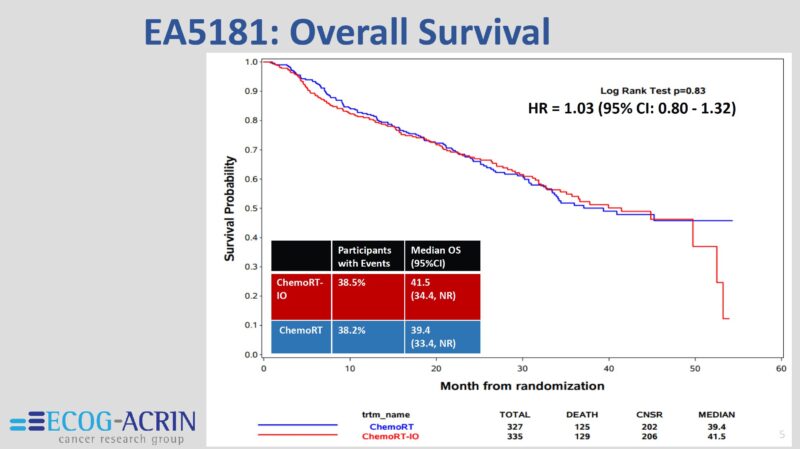
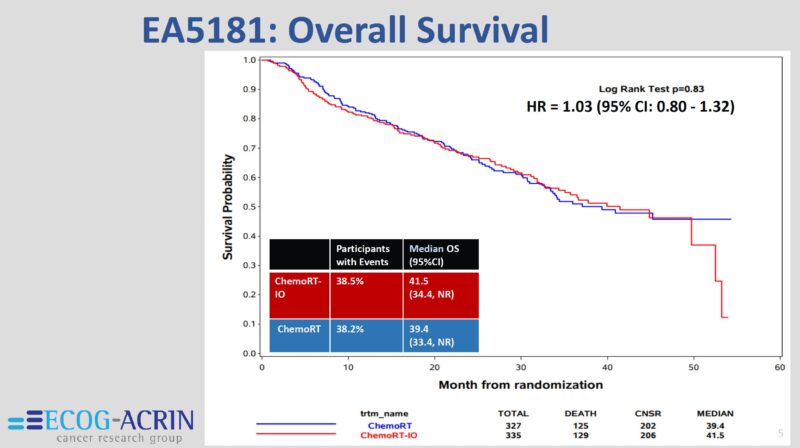
This contemporary series shows some of the highest OS rates ever reported in a study of this size for this population of patients with stage IIIA-IIIC, regardless of what local therapy was delivered to them. With a median f/u time of 30 months, the estimated 3y OS = 40%.
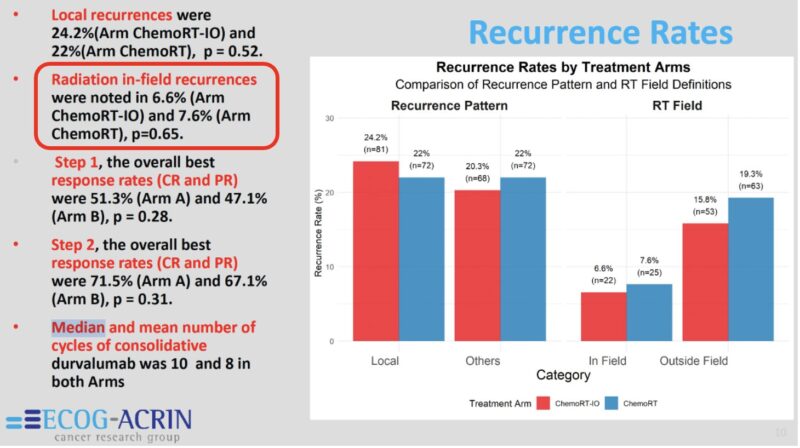
And what might be the biggest takeaway from this well-executed study is that the actual rates of in-field progression are actually discernible from the analysis. As shown in this slide, the in-field progression rates were <8%. That rate is invariably lower for stage II-IIIA NSCLC treated with CRT, and certainly not 20-50% as often quoted in MDT discussions.”
More posts featuring Drew Moghanaki.


