Parag Roy, Consultant Medical Oncologist at TMH & Meherbai TMH Jamshedpur, shared a post on LinkedIn:
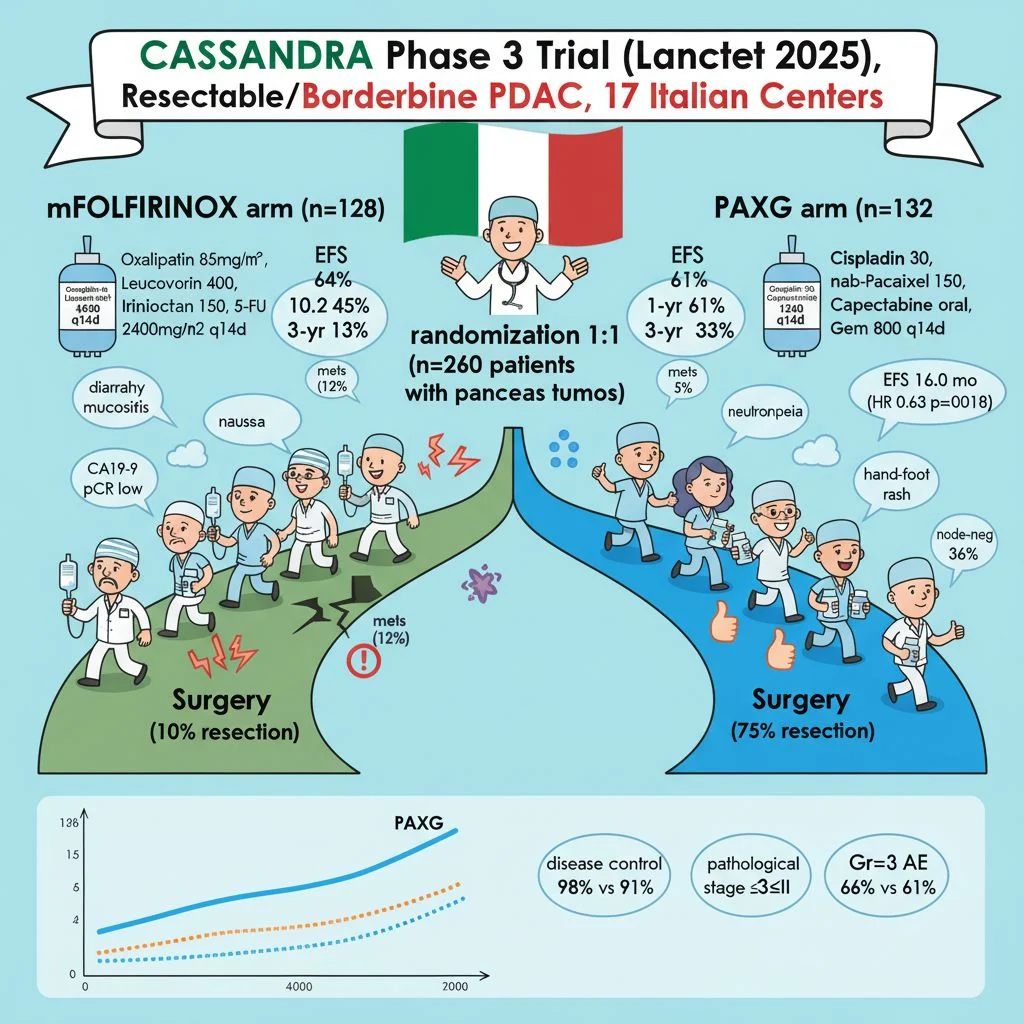
“Exciting results from the CASSANDRA trial (PACT-21) just published in The Lancet!
A phase 3 randomized study showing preoperative PAXG (cisplatin, nab-paclitaxel, capecitabine, gemcitabine) superior to mFOLFIRINOX for resectable/borderline resectable pancreatic ductal adenocarcinoma (PDAC). Median EFS: 16.0 vs 10.2 months (HR 0.63, p=0.0018).
Key highlights:
– Higher disease control (98% vs 91%), CA19-9 response (88% vs 64%), node-negative resections (36% vs 23%), fewer intraoperative mets (5% vs 12%)
– Comparable Gr≥3 toxicity (66% vs 61%)
– Sets PAXG as new standard for neoadjuvant therapy in this setting
Check out this fun cartoon summary I created – trial design, arms, efficacy, responses, side effects, and EFS graph all in one visual!(see the generated image above).
Thoughts on shifting to PAXG in practice? Let’s discuss!”
Title: Preoperative mFOLFIRINOX versus PAXG for stage I–III resectable and borderline resectable pancreatic ductal adenocarcinoma (PACT-21 CASSANDRA): results of the first randomisation analysis of a randomised, open-label, 2 × 2 factorial phase 3 trial
Authors: Michele Reni, Marina Macchini, Giulia Orsi, Letizia Procaccio, Giuseppe Malleo, Catia Carconi, Ilario Giovanni Rapposelli, Katia Bencardino, Mario Scartozzi, Gianpaolo Balzano, Domenico Tamburrino, Barbara Merelli, Elisa Sperti, Giulio Belfiori, Nicole Liscia, Silvia Bozzarelli, Mariacristina Di Marco, Emiliano Tamburini, Michele Milella, Sara Lonardi, Giorgio Ercolani, Michele Mazzola, Diego Palumbo, Valter Torri, Massimo Falconi
You Can Also Read: CASSANDRA-PACT-21 Trial Update: Preoperative PAXG vs mFOLFIRINOX in Resectable and Borderline Resectable PDAC



