The landscape of early-stage pancreatic ductal adenocarcinoma (PDAC) continues to evolve as clinicians move from surgery-first strategies toward perioperative treatment. Neoadjuvant and perioperative approaches have shown growing promise, yet no regimen had clearly surpassed the others. The CASSANDRA phase 3 trial changes this conversation.
For the first time, an intensive four-drug combination—PAXG (cisplatin, nab-paclitaxel, capecitabine, gemcitabine)—demonstrated superiority over modified FOLFIRINOX (mFOLFIRINOX), long considered a dominant regimen in advanced and early-stage PDAC. CASSANDRA directly compared the two in patients with stage I–III resectable or borderline resectable disease, providing high-level evidence for a new standard.
Methods
CASSANDRA is a randomized, open-label, multicenter phase 3 study conducted across 17 Italian academic hospitals. Eligible patients were 18–75 years old, had stage I–III PDAC confirmed histologically, and were classified as resectable or borderline resectable based on NCCN criteria, including biological high-risk features such as CA19-9 ≥500 IU/mL.
Patients were randomized 1:1 to receive four months of either PAXG or mFOLFIRINOX prior to planned surgery. Those who did not progress were later randomized again to receive two additional months of the same chemotherapy either before or after resection; only the first randomization is reported here.
The primary endpoint was event-free survival (EFS), defined as the first occurrence of radiologic progression, recurrence, unresectability, intra-operative metastases, sustained CA19-9 increase, or death. Secondary endpoints included radiologic and biochemical responses, pathological outcomes, recurrence patterns, safety, and quality of life. Analyses were performed in the intention-to-treat population (n=260).
Early CASSANDRA Findings at ASCO 2025
Preliminary results from the CASSANDRA phase III trial were first reported at the ASCO Annual Meeting on June 4, 2025, showing that neoadjuvant PAXG outperformed mFOLFIRINOX in resectable and borderline resectable PDAC. In 260 randomized patients, PAXG achieved a 3-year EFS of 30% compared with 14% for mFOLFIRINOX (HR 0.66). PAXG also produced higher disease control (98% vs 91%), deeper CA19-9 responses (88% vs 64%), and more favorable pathological downstaging (35% vs 23%). Resection rates were similar, and toxicity profiles reflected expected regimen differences, with more neutropenia on PAXG and more GI and neurologic toxicity on mFOLFIRINOX.
Lancet Full Results
The full data from the CASSANDRA trial were published in The Lancet on November 20, 2025. A total of 260 patients were included in the intention-to-treat population, with 132 assigned to PAXG and 128 to mFOLFIRINOX. Baseline characteristics were well balanced.
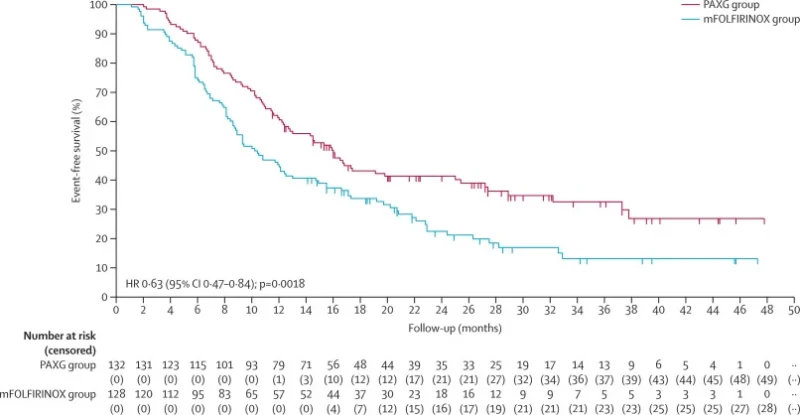
After a median follow-up of 28.5 months, PAXG significantly improved EFS, achieving 16.0 months versus 10.2 months with mFOLFIRINOX (HR 0.63; p=0.0018). One-year EFS was 61% for PAXG and 45% for mFOLFIRINOX; three-year EFS reached 33% versus 13%. The benefit was consistent across resectable and borderline resectable subgroups.
Key efficacy signals favoring PAXG included:
• Higher disease control (98% vs 91%)
• Stronger CA19-9 responses (88% vs 64%)
• More early pathological stages (IA–IB: 35% vs 23%)
• More node-negative resections (36% vs 23%)
• Fewer intra-operative or early postoperative metastases (5% vs 12%)
Resection and R0 rates were similar, indicating that PAXG’s advantage reflects deeper biological response rather than improved operability alone.
Safety
Severe toxicities were comparable between regimens, with grade ≥3 events occurring in 66% of PAXG patients and 61% of those receiving mFOLFIRINOX. Neutropenia was more common with PAXG; however, this did not translate into increased febrile neutropenia or infections.
mFOLFIRINOX produced more gastrointestinal and neurologic toxicity, including higher rates of nausea, diarrhea, neuropathy, paraesthesia, mucositis, and liver enzyme elevation. The only treatment-related death (sepsis) occurred in the mFOLFIRINOX arm.
Overall, both regimens were intensive but manageable in high-volume centers, with PAXG demonstrating a more favorable all-grade toxicity pattern.
Quality of Life
Quality-of-life assessments at baseline and month 4 were completed by 83% of PAXG patients and 64% of mFOLFIRINOX patients. More patients in the mFOLFIRINOX arm were unable to complete follow-up assessments due to early disease progression or toxicity, reflecting its lower disease control.
Across most EORTC QLQ-C30 and PAN26 domains, both regimens produced expected declines in fatigue, taste, weight-loss concerns, and hepatic symptoms. PAXG was associated with more leg weakness and body-image deterioration, while mFOLFIRINOX led to more pronounced reductions in role functioning, social functioning, nausea/vomiting, dry mouth, and activity planning. The overall trend suggests a more stable QoL trajectory with PAXG during neoadjuvant therapy.
Conclusion
The CASSANDRA (PACT-21) phase 3 trial shows that preoperative PAXG is superior to mFOLFIRINOX for stage I–III resectable and borderline resectable PDAC, with a clinically meaningful improvement in event-free survival, higher disease control and CA19-9 response rates, deeper pathological downstaging, and fewer intra-operative or early postoperative metastases—without an increase in grade ≥3 toxicity.
Overall survival data are still immature, but early results favor PAXG (median OS 32.1 vs 26.4 months). Taken together, these findings support preoperative PAXG as a standard option in this setting and a preferred comparator for future neoadjuvant trials in resectable and borderline resectable PDAC.

You can also read about Mitazalimab plus mFOLFIRINOX in Metastatic Pancreatic Cancer: Insights from the OPTIMIZE-1 Study on OncoDaily.