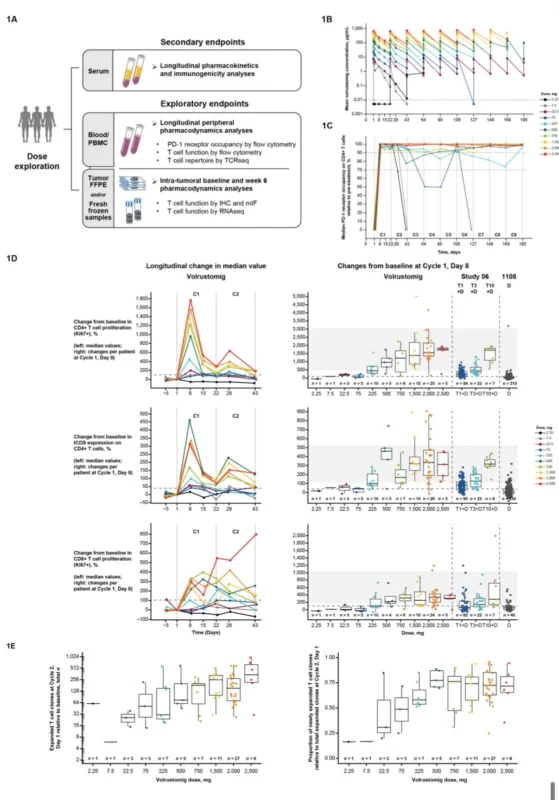
Aakash Desai, Assistant Professor and Associate Director of Phase 1 and Precision Oncology Program at the UAB O’Neal Comprehensive Cancer Center, shared a post on X:
“First-in-human study of volrustomig (PD-1/CTLA-4 bispecific) shows promising safety and early efficacy signals.
Key findings:
- Manageable safety profile
- Target engagement confirmed
- Early antitumor activity observed.”
Title: Safety, pharmacokinetics, pharmacodynamics, and preliminary efficacy from a first-in-human study of volrustomig, a novel PD-1/CTLA-4 bispecific antibody
Authors: Ben Tran, Mark Voskoboynik, Sang-We Kim, Charlotte Lemech, Enric Carcereny, Sun Young Rha, Myung-Ju Ahn, Enriqueta Felip, Ki Hyeong Lee, Eduardo Castañón Álvarez, James Chih-Hsin Yang, Paolo Antonio Ascierto, Mariano Provencio Pulla, Shunsuke Kondo, Yasutoshi Kuboki, Daniel Freeman, Xuyang Song, Jorge Blando, Steven Eck, Florian J. Song, Zoey Tang, Michael Kuziora, Shelby D. Gainer, Patrick Mitchell, Julie Asare, Amal Ayyoub, Ikbel Achour, Deepa S. Subramaniam, Seock-Ah Im
Read The Full Article

Other articles featuring Aakash Desai on OncoDaily.


