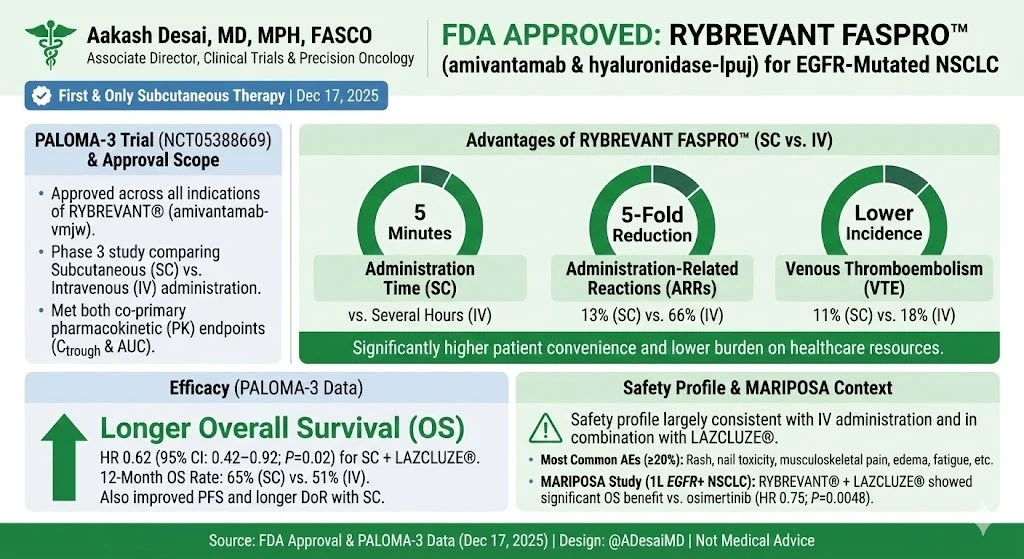
Aakash Desai, Associate Director, Phase 1 and Precision Oncology Program at UAB O’Neal Comprehensive Cancer Center, shared a post on X:
“FDA approval update in EGFR-Mutant NSCLC!
FDA approves Rybrevant FASPRO (amivantamab + hyaluronidase) plus lazertinib for first-line EGFR-mutant NSCLC.
- SC administration shorter
- Simpler dosing
- Meaningful step forward for clinic workflow and patient experience
Delivery matters—this is progress! Also excited for the reduced time toxicity on patients!”
More posts featuring Aakash Desai on OncoDaily.


