Aakash Desai, Associate Director, Phase 1 and Precision Oncology Program at UAB O’Neal Comprehensive Cancer Center, shared a post on X by Sanad Alhushki, MD Research fellow at UAB O’Neal Comprehensive Cancer Center, adding:
“Dont miss it!
Sanad Alhushki and I will try to decode some of the great advances in ADCs for Lung Cancer really looking forward to this discussion!!”
Quoting Sanad Alhushki’s post:
“Setting the Stage (2L NSCLC)
1L NSCLC has transformed with chemo-IO combos
2L landscape in non-driver dz remains challenging
Historically, doce has been backbone
ADCs are reshaping field
2L tx for NSCLC = rapidly evolving space
Setting the Stage (2L NSCLC)
Tonight, we’ll review 2 ADCs
vedotin (Teliso-V) – c-MET–directed
Trastuzumab deruxtecan (T-DXd) – HER2-directed
Crucial for real-world decision-making.
ADCs in NSCLC
Ag recognition → Ab binds tumor-specific target
Internalization into tumor cell
Payload release into cytosol
Cytotoxic activity (MT disruption/DNA damage)
Increased precision, reduced off-target tox
A Case: Pat
Pat was dx w/ non-driver met adenocarcinoma, PD-L1 5%
1L: carbo+pemetrexed+pembro → pembro maint (~1 y)
Now, radiographic PD
Classic real-world scenario: next-line choices matter
What Would You Do Next?
Q. At PD on chemo-IO, what’s your next step Biomarker
test+liquid bx
CT monotx
Continue pembro
Re-image in 6–8w
1 correct answer…let’s see what Oncology Twitter community thinks.
T-DXd: major option in HER2-altered NSCLC—but understanding which HER2 biomarker matters is critical
HER2 alterations include:
HER2mut – ex20ins most common
HER2amp
HER2 O-E (IHC 2+/3+)
HER2 alterations in NSCLC
Not all HER2 alterations behave the same
HER2mut respond best to T-DXd (DESTINY-Lung02)
HER2 O-E → evolving space.
HER2 alterations in NSCLC
Before considering T-DXd, ensure:
NGS-based HER2mut testing
HER2 IHC (later-line or at PD)
Repeat profiling at PD (ctDNA + tissue if feasible).

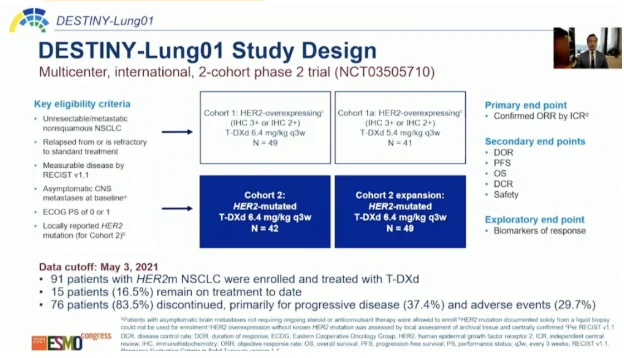
Phase 2 DESTINY-Lung01 Study of T-DXd
- HER O-E (IHC 2+ or 3+) → 6.4 mg/kg Q3W (n = 49) → 5.4 mg/kg Q3W (n = 41)
- HER2mut → 6.4 mg/kg Q3W (n = 91).
Ph2 DL-01 — Key Takeaways
HER2mut: ORR ~55% with durable responses
HER2-OE: Modest activity overall; strongest signal in IHC 3+ Responses can be deep & rapid, especially in ex20ins
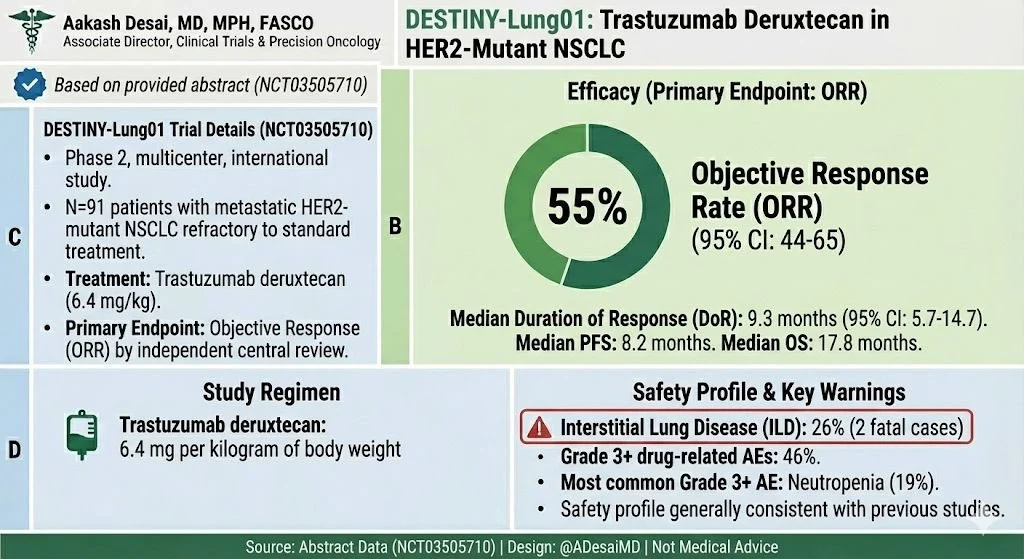
Ph2 DL-01: Safety
ILD: 26%, most Gr 1/2 (Gr 5, n = 2)
Most common Gr 3+ AE: neutropenia
Safety consistent with prior studies
Tox profile includes ILD/pneumonitis→needs close monitoring.
Phase 2 DESTINY-Lung01 Study of T-DXd
Takeaways:
T-DXd changed tx landscape, specifically for HER2mut NSCLC
Paved the way for 5.4 mg/kg adoption (DESTINY-Lung02)
Teliso-V & MET testing
Shifting gears to Teliso-V
Teliso-V targets MET overexpression (MET-OE) NOT METex14 skipping mut.
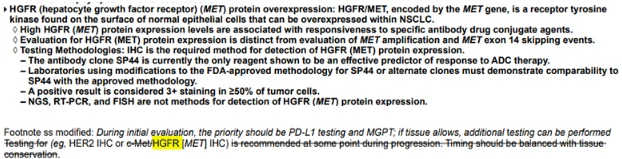
Teliso-V & MET Testing
- MET IHC (SP44 clone) required
- MET-OE=3+ staining in ≥50% tumor cells
- Molecular METamp≠MET OE
- NGS/FISH can’t sub for MET IHC for ADC eligibility
Biomarker-driven ADC→MET IHC accuracy is essential.
MET IHC (SP44):
- Biomarker for Teliso-V SP44 is the only validated Ab predicting Teliso-V response.
- MET O-E is distinct from METamp.
- METex14 skipping = separate pathway → treated with TKIs (capmatinib, tepotinib).
MET IHC (SP44): Biomarker for Teliso-V
Order at PD / when considering ADC eligibility
Use FDA-aligned staining method
MET IHC 3+ required 4 optimal response prediction
Bottom line: Without proper MET IHC testing, pts may miss entire tx class.
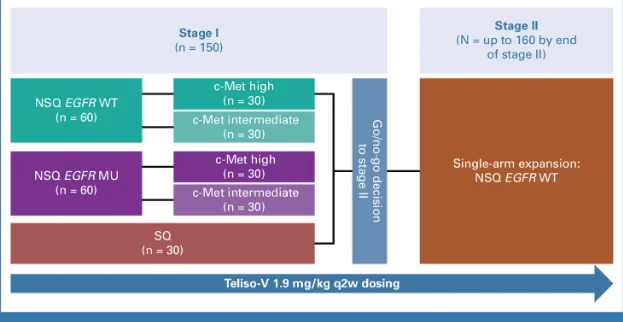
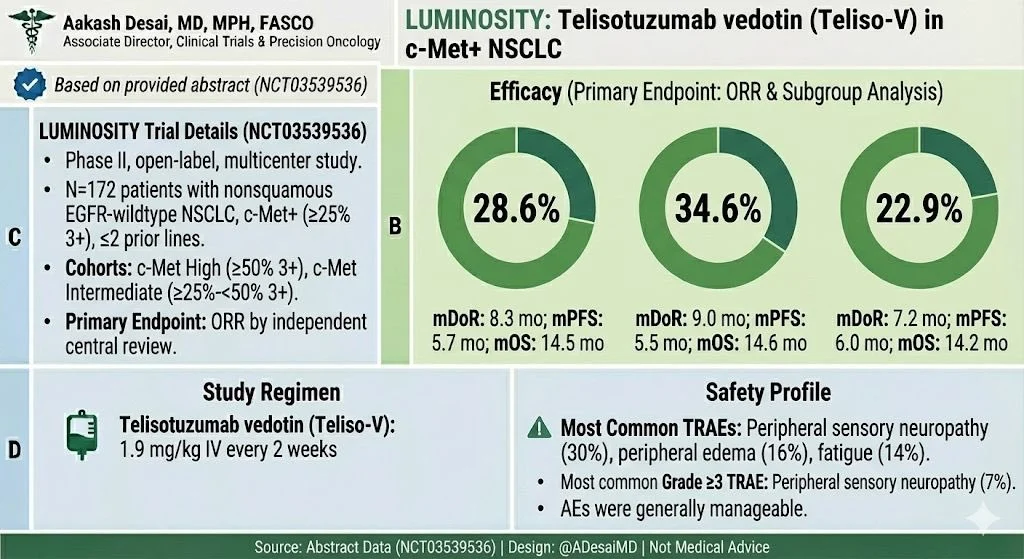
Ph 2 LUMINOSITY Study of Teliso-V for MET O-E mNSCLC
≤ 2 prior LOT
Pts received Teliso-V 1.9 mg/kg Q2W
Primary endpoint: ORR by ICR
N = 172 EGFRwt, nonsq.
LUMINOSITY Study: Key Efficacy Data
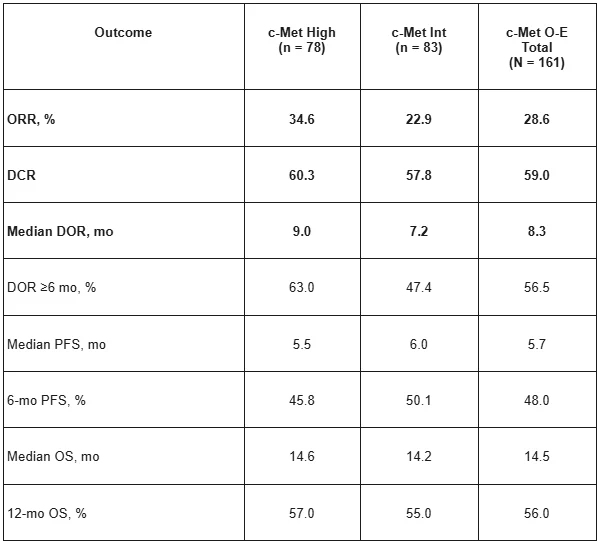
Results in MET-high (IHC 3+) population:
- ORR 35% in MET-high nonsq NSCLC.
- Responses enriched in IHC 3+.
- Durable disease control.
- Sq. cohort: lower activity → underscores BM importance.
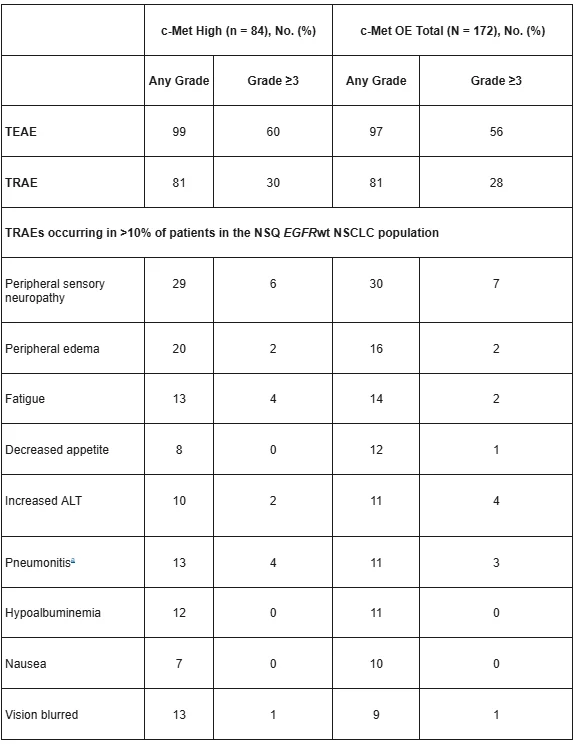
LUMINOSITY Study: Safety Data
Most common Any Gr TRAEs: PSN, PE, fatigue
Gr ≥ TRAE: 28%
ILD: 10%, most Gr 1/2
AE D/C: 22% (ILD/pneumonitis 9%, neuropathy 9%)
Gr 5 events: n = 3
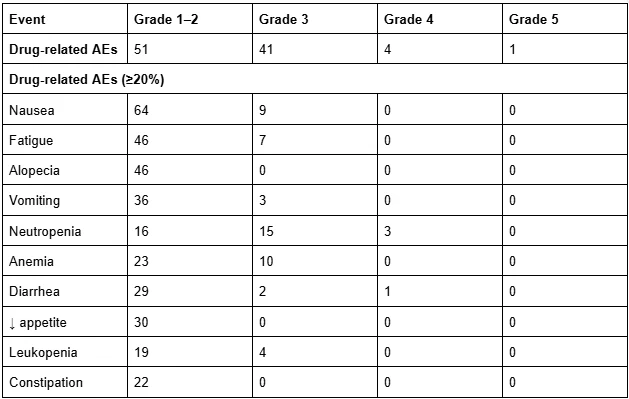
LUMINOSITY (c-Met+ NSCLC) — Telisotuzumab vedotin (Teliso-V)
Key Takeaways
- ORR: 28.6% (overall)
- c-Met High: 34.6%
- c-Met Intermediate: 22.9%
- mDoR: 7.2–9.0 mo
- mPFS: 5.5–6.0 mo
- mOS: 14–15 mo
AEs: Sensory neuropathy (30%), edema (16%), fatigue (14%); G3 neuropathy 7%
Another ADC option in 2L space for c-Met+ NSCLC—reinforces need for precise MET IHC testing.
Back to Our Case: Pat
Pat receives an ADC. How should we monitor for ILD?
Our Case: Pat
Pat develops asymptomatic Gr 1 ILD while on T-DXd
What do you do
Hold tx
Hold tx, start steroid
D/C tx
Other
Do you resume T-DXd at resolution of ILD.
What are your key takeaways from this discussion
❖ADCs reshaping 2L mNSCLC tx
❖Not all alterations within a gene behave the same
❖ proper BM testing is critical
T-DXd – HER2mut & HER2 IHC 3+
Teliso-V – MET O-E by IHC.”
More posts featuring Aakash Desai on OncoDaily.


