Aakash Desai, Associate Director, Phase 1 and Precision Oncology Program at UAB O’Neal Comprehensive Cancer Center, shared a post on LinkedIn:
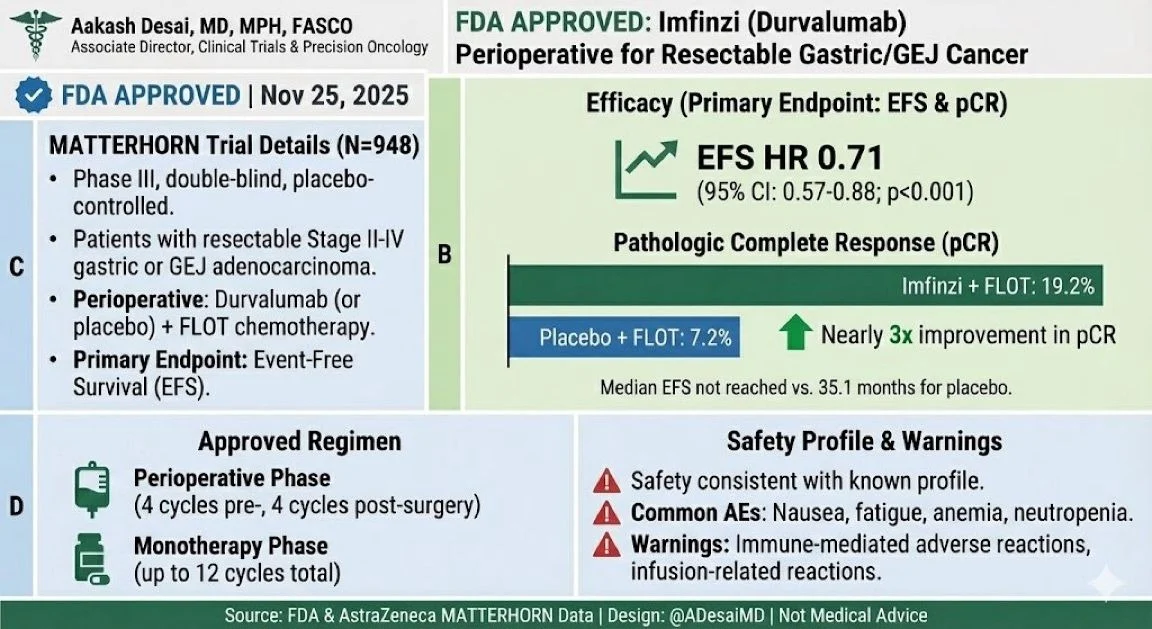
“FDA has now approved perioperative Durvalumab + FLOT for resectable gastric and GEJ adenocarcinoma, based on the MATTERHORN results!
A major step forward—immunotherapy enters the curative setting, now in gastric/GEJ!”
More posts on Immunotherapy.


