Aakash Desai, Associate Director, Phase 1 and Precision Oncology Program at UAB O’Neal Comprehensive Cancer Center, shared a post on LinkedIn:
“Big step forward for precision oncology – with randomized evidence!
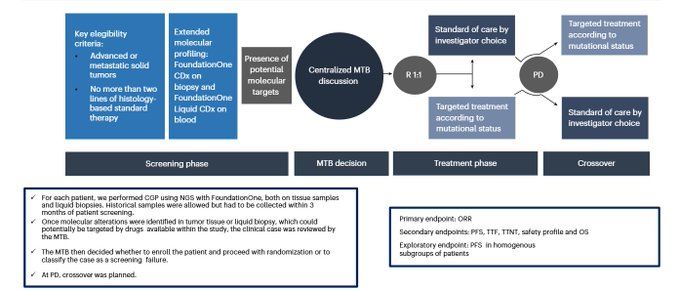
The ROME trial, a multicenter phase 2 RCT, provides some of the most robust data yet supporting tumor-agnostic, genomics-guided treatment.
In patients with advanced solid tumors (n=400) post 1–2 prior therapies, molecular tumor board-guided tailored therapy (TT) was compared to standard of care (SoC).
Key findings:
- ORR: 17.5% (TT) vs 10% (SoC) – P = 0.029
- Median PFS: 3.5 vs 2.8 months – HR 0.66, P = 0.0002
- 12-month PFS: 22% vs 8.3%
- Grade 3/4 AEs: 40% vs 52%
- OS was similar—possibly influenced by a 52% crossover rate
These results offer some of the strongest support yet for the clinical benefit of tumor-agnostic, biomarker-driven approaches in real-world, diverse patient populations.”
Title: Genomically matched therapy in advanced solid tumors: the randomized phase 2 ROME trial
Authors: Paolo Marchetti, Giuseppe Curigliano, Mauro Biffoni, Sara Lonardi, Simone Scagnoli, Lorenzo Fornaro, Valentina Guarneri, Ugo De Giorgi, Paolo Antonio Ascierto, Giovanni Blandino, Giulia D’Amati, Massimo Aglietta, Chiara Cremolini, Pierfranco Conte, Edoardo Crimini, Maurizio Ceracchi, Simona Pisegna, Sofia Verkhovskaia, Roberto Bordonaro, Sergio Bracarda, Giovanni Butturini, Lucia Del Mastro, Andrea DeCensi, Agnese Fabbri, Elisabetta Fenocchio, Stefania Gori, Giulio Metro, Annamaria Pessino, Daniele Pozzessere, Fabio Puglisi, Stefano Tamberi, Alberto Zambelli, Donatella Marino, Ettore Capoluongo, Federico Cappuzzo, Bruna Cerbelli, Giuseppe Giannini, Umberto Malapelle, Federica Mazzuca, Marianna Nuti, Giancarlo Pruneri, Maurizio Simmaco, Lidia Strigari, Giuseppe Tonini, Nello Martini, Andrea Botticelli
Read the Full Article in nature medicine.

More posts featuring Aakash Desai on OncoDaily.


