
Photo taken from
Udhayvir Grewal/X
Jun 10, 2024, 10:02
Udhayvir Grewal: Real world post-marketing pharmacovigilance analysis on hematological toxicities in the US
Udhayvir Grewal, Resident Physician at Ochsner LSU Health Shreveport, recently shared a post on X:
“Hot off the press.
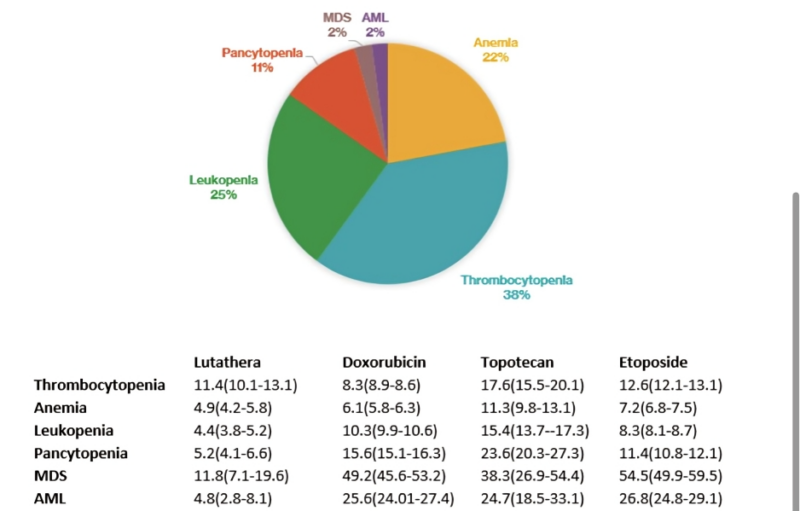
Happy to share our real-world post-marketing pharmacovigilance analysis on hematological toxicities (focus on MDS/AML) with Lutathera (Lu177-DOTA-TATE) in the US.
- Post-marketing surveillance data from the U.S. FDA (2018-2023)
- 3443 adverse events, 243 (7.1%) were hematologic
- Majority thrombocytopenia, leukopenia, anemia, and pancytopenia
- MDS and AML comprised 2% of the reported events each.
- For context, we compare the reporting of hematological toxicities (esp MDS and AML) with topoisomerase inhibitors
- This is a helpful reference that may potentially inform discussions with patients planning to receive PRRT (now referred to as radioligand therapy or RLT).
Limitations – captures only the US data and the events reported to the U.S. FDA.
Thank you Anuj Thakre for your help with the project.”
Read further
Source: Udhayvir Grewal/X
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
Aug 25, 2025, 12:37
Aug 25, 2025, 12:23
Aug 25, 2025, 11:07
Aug 25, 2025, 09:45
