
FDA Breakthrough Therapy Designation for Iza-bren: Advancing Treatment for EGFR-Mutant NSCLC
Izalontamab brengitecan (Iza-bren), developed by SystImmune Inc. in collaboration with Bristol Myers Squibb, has received Breakthrough Therapy Designation (BTD) from the U.S. Food and Drug Administration (FDA). This designation is granted for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutations, specifically exon 19 deletions or exon 21 L858R substitutions, in patients whose disease has progressed following treatment with an EGFR tyrosine kinase inhibitor (TKI) and platinum-based chemotherapy.
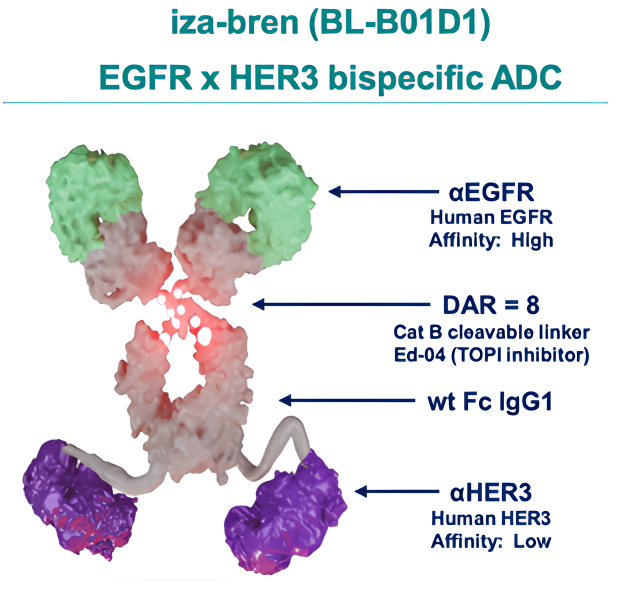
Iza-bren, a potential first-in-class bispecific antibody-drug conjugate (ADC), targets both EGFR and HER3, which are associated with cancer cell proliferation. The ADC’s payload is a topoisomerase 1 inhibitor, designed to cause cancer cell death.
This breakthrough designation underscores the promising clinical data suggesting Iza-bren’s potential to address the significant unmet need for advanced NSCLC patients who no longer benefit from EGFR TKIs or platinum-based chemotherapy. The FDA’s BTD accelerates the development of drugs that show significant promise over current treatment options.

“The FDA’s granting of Breakthrough Therapy Designation underscores the potential of iza-bren to meaningfully improve clinical outcomes for patients with previously treated epidermal growth factor receptor mutation NSCLC. The data we have generated to date suggest that iza-bren could address a critical unmet need in patient care, and we look forward to working closely with the FDA to conduct the relevant clinical studies and seek regulatory approval.”
said Dr. Jonathan Cheng, Chief Medical Officer of SystImmune.
How Iza-bren Works to Treat EGFR-Mutant NSCLC
Iza-bren (izalontamab brengitecan) is a bispecific antibody-drug conjugate (ADC) that targets both epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 3 (HER3), which are frequently overexpressed in various epithelial cancers, including non-small cell lung cancer (NSCLC). EGFR plays a critical role in promoting cell proliferation, while HER3 is involved in activating downstream signaling pathways that support cancer cell survival and resistance to treatment.
Source: bydrug.pharmcube.com
Iza-bren works by binding to both of these receptors simultaneously, blocking their signaling and reducing the proliferative and survival signals to cancer cells. The ADC’s topoisomerase 1 inhibitor payload is released upon internalization of the antibody-receptor complex, inducing DNA damage and genotoxic stress within the cancer cells. This leads to cell cycle arrest and apoptosis (programmed cell death).
Through this dual-target mechanism, Iza-bren offers a powerful approach to overcoming resistance mechanisms associated with EGFR-targeted therapies, providing a novel treatment option for patients with EGFR-mutant NSCLC who have progressed after previous treatments such as EGFR tyrosine kinase inhibitors (TKIs) and platinum-based chemotherapy.
What is Breakthrough Therapy Designation ?
Breakthrough Therapy Designation (BTD) is a program developed by the U.S. Food and Drug Administration (FDA) to expedite the development and review of drugs aimed at treating serious conditions. It is granted when preliminary clinical evidence shows that a drug may offer substantial improvement over existing therapies, particularly in clinically significant endpoints such as irreversible morbidity, mortality, or serious disease symptoms. The drug must address an unmet need and show promise in significantly improving treatment outcomes, either through the magnitude of its effect or the importance of its clinical impact.

Key features of Breakthrough Therapy Designation (BTD) include the eligibility criteria, where the drug must target a serious condition and demonstrate substantial improvement over existing therapies. Clinically significant endpoints are essential, which may include irreversible morbidity, mortality, serious symptoms, surrogate endpoints, pharmacodynamic biomarkers, or a significantly improved safety profile.
The benefits for the drug are extensive: it includes Fast Track features for more frequent interactions with the FDA, intensive guidance on drug development starting as early as Phase 1, and a commitment from senior FDA management to prioritize the drug’s development.
By streamlining the review process, BTD helps accelerate the availability of promising therapies, improving treatment options and addressing unmet medical needs.
Clinical Trials Supporting Breakthrough Designation
The FDA’s decision was based on data from three ongoing clinical trials:
BL-B01D1-101
The BL-B01D1 Phase I Clinical Study is designed to evaluate the safety, tolerability, pharmacokinetics, and preliminary efficacy of BL-B01D1 in patients with locally advanced or metastatic solid tumors. This study is conducted in two phases: Phase Ia and Phase Ib.
Phase Ia:
The focus is on investigating the safety and tolerability of BL-B01D1 in patients with locally advanced or metastatic solid tumors.
The study aims to determine the dose-limiting toxicity (DLT) and maximum tolerated dose (MTD) of BL-B01D1. This phase will help identify the appropriate dose range for the next phase of the trial.
Phase Ib:
In this phase, BL-B01D1 will be administered at the recommended dose from Phase Ia, and the safety and tolerability will be further investigated.
The goal of Phase Ib is to determine the recommended Phase II dose (RP2D), which will be used in later stages of clinical testing.
Primary Objectives
- Determine the dose-limiting toxicity (DLT) and establish the maximum tolerated dose (MTD) of BL-B01D1 in Phase Ia.
- In Phase Ib, confirm the recommended Phase II dose (RP2D) for subsequent studies.
Secondary Objectives
- Preliminary Efficacy: Evaluate the initial efficacy of BL-B01D1 in patients with locally advanced or metastatic solid tumors, using metrics such as Objective Response Rate (ORR).
- Pharmacokinetics: Investigate the pharmacokinetic characteristics of BL-B01D1 (absorption, distribution, metabolism, and excretion).I
- mmunogenicity: Assess the immunogenicity (immune response) of BL-B01D1 in patients to ensure there are no adverse immune reactions.
The primary goal of this study is to establish the safety profile and optimal dose of BL-B01D1, with subsequent evaluation of its efficacy and pharmacokinetic characteristics. The findings from this study will be used to determine the dose for future Phase II trials.
Results:
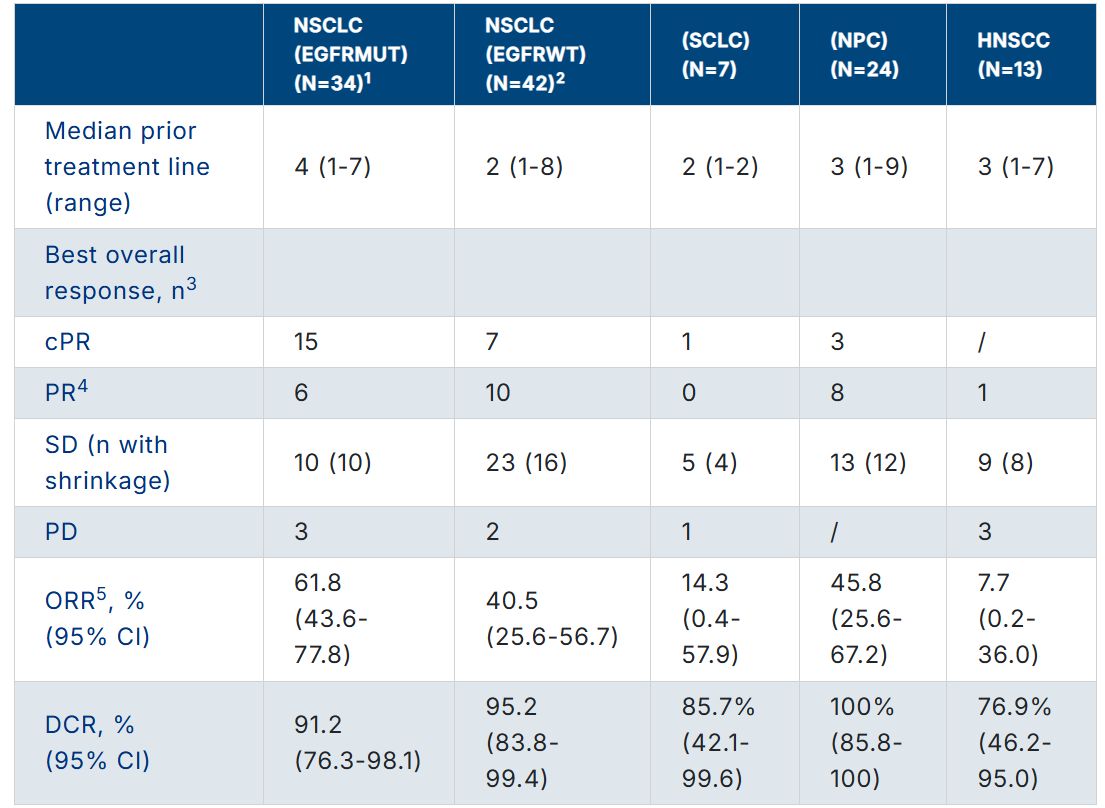
Source: ASCO 2023 Annual Meeting Abstract
BL-B01D1-203:
The BL-B01D1-203 Phase 2 study is evaluating the combination of iza-bren (BL-B01D1) and osimertinib (Tagrisso) in patients with locally advanced or metastatic NSCLC.Patients aged 18+ with measurable lesions and confirmed NSCLC are treated with iza-bren plus osimertinib for a 3-week cycle. If clinical benefit is observed, treatment continues; otherwise, it is discontinued due to progression, toxicity, or other reasons.
- The primary endpoints include determining the optimal dose (RP2D) and assessing the Objective Response Rate (ORR).
- Secondary endpoints focus on Progression-Free Survival (PFS), Disease Control Rate (DCR), Duration of Response (DOR), and Treatment-Emergent Adverse Events (TEAEs).
This study seeks to determine if this combination provides a safe and effective treatment for patients who have progressed on previous therapies.
BL-B01D1-LUNG-101
The BL-B01D1-LUNG-101 study is a global, multi-center, Phase 1 clinical trial designed to evaluate the safety, tolerability, pharmacokinetics, and initial efficacy of BL-B01D1 (iza-bren), a bispecific antibody-drug conjugate (ADC), in participants with metastatic or unresectable non-small cell lung cancer (NSCLC) and other solid tumors.
Study Design
- Cohort A: Participants will be dosed on Day 1 and Day 8 of a continuous 21-day treatment cycle.
- Cohort B: Participants will be dosed on Day 1 of a continuous 21-day treatment cycle.
The primary objectives of the study are to:
- Assess the safety and tolerability of BL-B01D1.
- Characterize its pharmacokinetics.
- Evaluate its preliminary efficacy in patients with EGFR-mutant NSCLC who have progressed after previous treatments.
The trial will also look at the pharmacodynamic effects and any biomarker correlations related to the treatment’s effectiveness.
Conclution
Izalontamab brengitecan (Iza-bren) represents a promising new treatment option for patients with locally advanced or metastatic NSCLC harboring EGFR mutations, particularly for those who have progressed after prior therapies. The FDA’s Breakthrough Therapy Designation highlights the potential of Iza-bren to address a significant unmet need in this patient population.
Ongoing clinical trials, such as BL-B01D1-101, BL-B01D1-203, and BL-B01D1-LUNG-101, are pivotal in evaluating the safety, efficacy, and pharmacokinetics of Iza-bren, with early data suggesting its ability to overcome resistance mechanisms associated with traditional EGFR-targeted therapies. As the study progresses, Iza-bren could significantly enhance treatment outcomes and provide a new, targeted therapeutic option for patients with advanced NSCLC.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
