Christine Hodgdon: Project 5 in 5 is a crowdsourcing initiative from FDA’s Oncology Center of Excellence
Christine Hodgdon, Stage IV Breast Cancer Survivor and Advocate treated at Johns Hopkins, shared a post by FDA Oncology, on X/Twitter, adding:
”This is a very cool way to share your voice! Project 5 in 5 is a crowdsourcing initiative from FDA’s Oncology Center of Excellence to identify 5 clinically relevant questions that can be answered through use of pragmatic clinical trials over the next 5 years.”
Quoting FDA Oncology’s post:
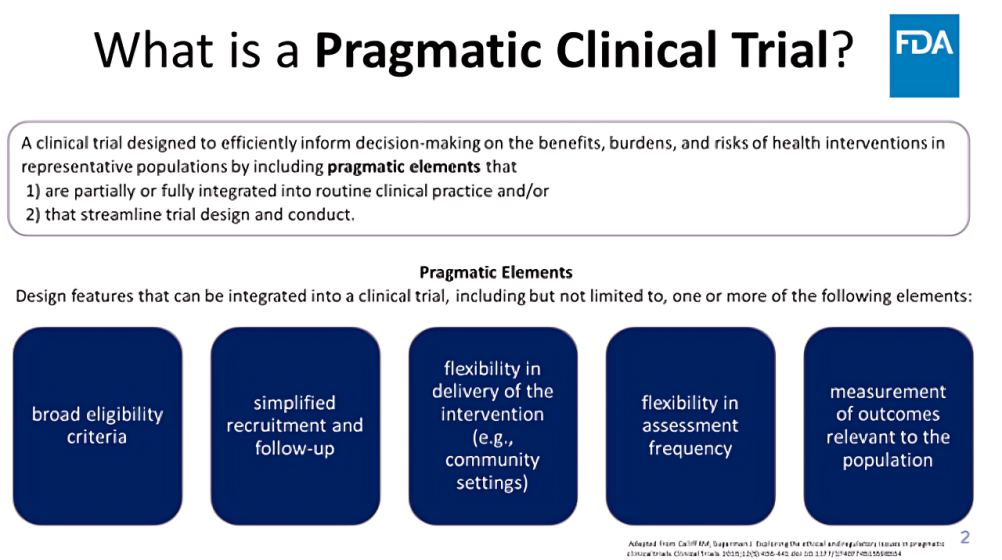
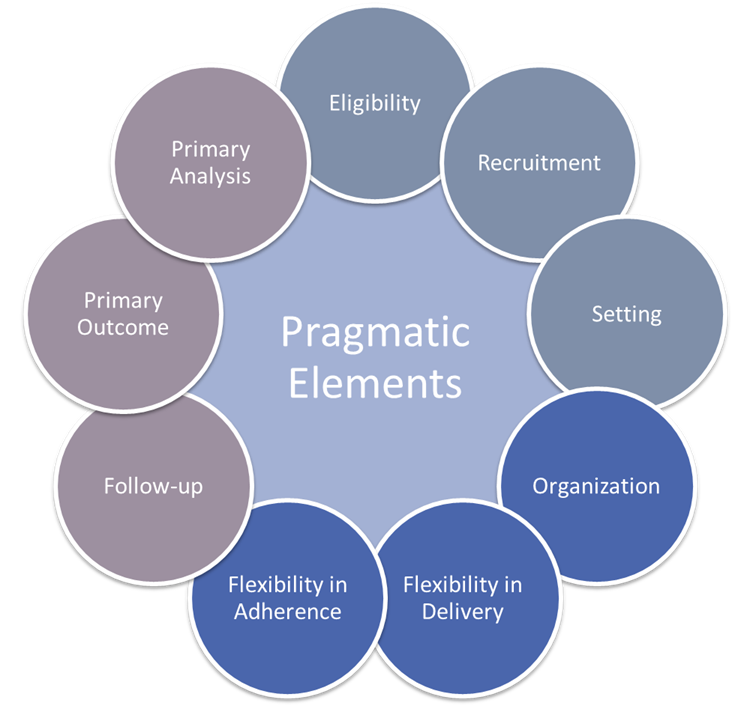
”What are pragmatic clinical trials? Clinical trials that streamline data collection and design by collecting only necessary information to answer the research question.
Pragmatic clinical trials:
- Incorporate design elements more reflective of routine clinical practice.
- Streamline data collection and study design by collecting only data necessary to answer the question.
- Use most straightforward endpoint feasible (i.e., overall survival).
- Decrease burden for trial participants, investigators, sponsors.
- Improve trial participation, increase diversity, less-restrictive eligibility criteria, rapid enrollment, reduce attrition.
- Functional efficiencies and flexibility in trial delivery and outcome measurement.
- Generate evidence representative of the general population affected by the clinical question.
Learn more about pragmatic clinical trials and take part in Project 5 in 5 to crowdsource ideas for pragmatic trials in oncology! Sign up and submit ideas here.”
Source: Christine Hodgdon/X and FDA Oncology/X
The Oncology Center of Excellence (OCE) was given authorization through the 21st Century Cures Act of 2016 and formally founded on January 19, 2017. This Center brings together specialists from across the FDA to facilitate accelerated evaluations of medical products intended for the treatment of oncologic and hematologic malignancies. The OCE also leads numerous initiatives aimed at advancing both the research and regulatory processes related to medical products for individuals affected by cancer.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
