
Neuroblastoma: What patients should know about
What is Neuroblastoma?
Neuroblastoma is a type of cancer that originates in early nerve cells, known as neuroblasts, and is most commonly found in infants and young children. It typically begins in the adrenal glands but can also develop in various areas such as the head, neck, chest, abdomen, or spine.
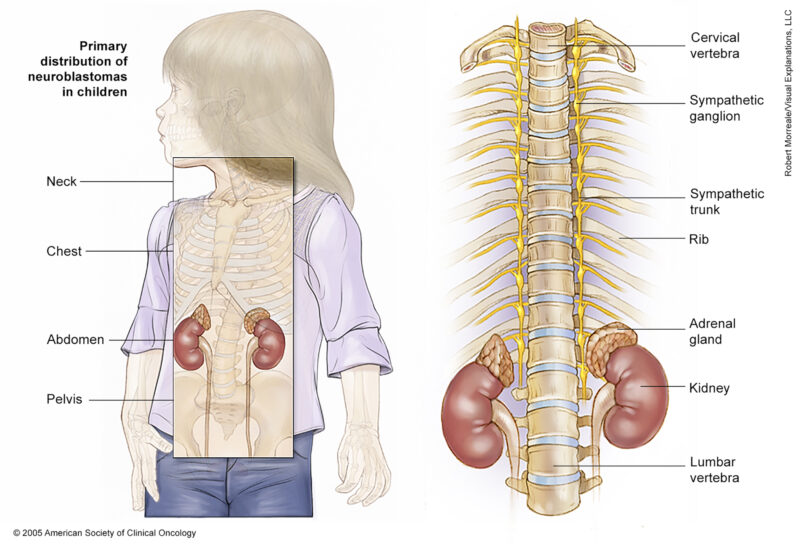
Source of image – Cancer.net
Causes and Risk Factors of Neuroblastoma
- Age – Neuroblastoma is most common in infants and young children, with it being very rare in people over 10 years old.
- Heredity – While most cases are not inherited, about 1-2% of disease cases have a family history of the disease. Children with familial neuroblastoma tend to be diagnosed at a younger age.
- Birth defects – Some studies have shown an increased risk of disease in children with certain congenital anomalies or birth defects. This may be related to genetic changes during fetal development.
- Prenatal and perinatal factors – Maternal anemia during pregnancy, neonatal respiratory distress, and low Apgar scores at birth have been associated with increased cancer risk, particularly for cases diagnosed before 1 year of age.
Symptoms of Neuroblastoma
- lump in the abdomen, neck, or chest
- bone pain
- swollen stomach and trouble breathing (in infants)
- bulging eyes
- dark circles around the eyes (“black eyes”)
- painless, bluish lumps under the skin (in infants)
- weakness or paralysis 2 (loss of ability to move a body part)
Less common signs and symptoms of cancer include the following:
- fever
- shortness of breath
- feeling tired
- easy bruising or bleeding
- petechiae (flat, pinpoint spots under the skin caused by bleeding)
- high blood pressure
- severe watery diarrhea
- Horner syndrome (droopy eyelid, smaller pupil, and less sweating on one side of the face)
- jerky muscle movements
- uncontrolled eye movements (opsoclonus-myoclonus syndrome, or Dancing Eyes-Dancing Feet Syndrome)
These and other signs and symptoms may be caused by neuroblastoma or by other conditions. The only way to know is to see your child’s doctor. The doctor will ask you when the symptoms started and how often your child has had them as a first step in making a diagnosis.
Diagnosis of Neuroblastoma
- Urine catecholamine studies: A test in which a urine sample is checked to measure the amounts of certain substances, vanillylmandelic acid (VMA) and homovanillic acid (HVA), that are made when catecholamines break down and are released into the urine. A higher-than-normal amount of VMA or HVA can be a sign of cancer.
- Blood chemistry studies: A test in which a blood sample is checked to measure the amounts of certain substances released into the blood by organs and tissues in the body. A higher-than-normal lactate dehydrogenase (LDH) can be a sign of disease.
- Ferritin level: A test in which a blood sample is checked to measure the amount of ferritin (a protein that stores iron in cells). A higher-than-normal amount may be a sign of disease.
- MIBG scan: A procedure to find neuroendocrine tumors, such as neuroblastoma. A very small amount of a substance called radioactive MIBG is injected into a vein and travels through the bloodstream. Neuroendocrine tumor cells take up the radioactive MIBG and are detected by a scanner. Scans may be taken over 1-3 days. An iodine solution may be given before or during the test to keep the thyroid gland from absorbing too much of the MIBG. This test is also used to find out how well the tumor is responding to treatment. MIBG is also used in high doses to treat cancer.
- CT scan (CAT scan): A procedure that makes a series of detailed pictures of areas inside the body, taken from different angles. The pictures are made by a computer linked to an x-ray machine. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
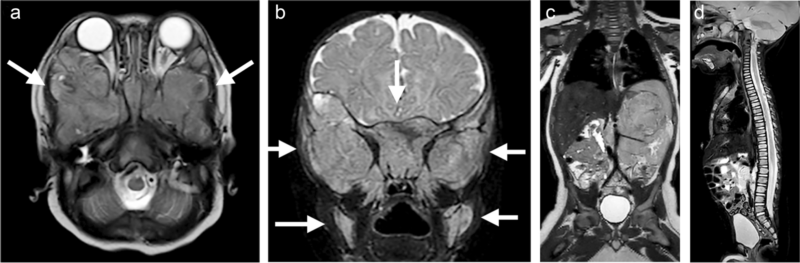
A 5-month-old boy with raccoon eyes (periorbital ecchymosis) due to extensive bone marrow metastasis from abdominal neuroblastoma. An axial T2-weighted image (a) and a coronal short tau inversion recovery (STIR) image (b) of the skull base show extensive bone marrow metastases around both orbits (arrows). c A coronal T2-weighted image shows the primary tumor arising from the left adrenal gland with vascular encasement of the renal pedicle (Image Defined Risk Factors positive). d A sagittal STIR image illustrates diffuse bone marrow metastasis of the spine.
Treatment
This challenging pediatric cancer originating from neural crest cells requires a multifaceted treatment approach tailored to the individual patient’s risk group, stage of disease, and genetic characteristics. The management of disease involves a combination of surgical interventions, chemotherapy, radiation therapy, targeted therapies, immunotherapy, and stem cell transplantation. Each treatment modality plays a crucial role in addressing the complex nature of cancer and improving patient outcomes
Surgery
Surgical resection of the primary tumor is a cornerstone of treatment, particularly for low-risk and intermediate-risk diseases. Complete surgical removal of the tumor can significantly impact the prognosis and reduce the tumor burden, leading to improved outcomes. In cases where complete resection is not feasible, debulking surgery may be performed to reduce the size of the tumor and facilitate subsequent treatments. Surgical interventions are often complemented by other modalities to achieve optimal disease control.
Chemotherapy
Chemotherapy is a fundamental component of treatment, especially for intermediate-risk and high-risk diseases. Chemotherapeutic agents, such as cisplatin, etoposide, doxorubicin, and cyclophosphamide, are commonly used to target cancer cells and reduce tumor size before surgery. High-dose chemotherapy regimens, followed by autologous stem cell transplantation, are often employed for high-risk groups to eradicate residual disease and improve long-term survival rates. Chemotherapy plays a critical role in inducing tumor regression, controlling metastatic spread, and preparing patients for subsequent treatments.
Radiation Therapy
Radiation therapy may be utilized in the management of cancer to target residual tumor cells after surgery or to treat metastatic sites. External beam radiation therapy delivers high-energy beams to the tumor site, destroying cancer cells and preventing local recurrence. Radiation therapy is particularly beneficial for patients with unresectable tumors, residual disease after surgery, or metastases to bone or other organs. Careful planning and precise delivery of radiation are essential to minimize side effects and preserve healthy tissues․
Targeted Therapies
Advancements in molecular profiling and genetic testing have led to the development of targeted therapies for disease. Agents that specifically target molecular pathways involved in tumor growth and survival, such as ALK inhibitors (e.g., crizotinib) and anti-GD2 monoclonal antibodies (e.g., rituximab), have shown promising results in high-risk neuroblastoma. Targeted therapies aim to exploit the vulnerabilities of cancer cells while sparing normal tissues, leading to more effective and less toxic treatment options for patients with specific genetic alterations.
Immunotherapy
Immunotherapy has emerged as a promising treatment strategy for disease, harnessing the power of the immune system to target and eliminate cancer cells. Monoclonal antibodies, such as rituximab, target GD2, a surface antigen expressed on neuroblastoma cells, enhancing immune recognition and destruction of tumor cells. Chimeric antigen receptor (CAR) T-cell therapy, cancer vaccines, and cytokine-based therapies are also being investigated for their potential to enhance the immune response against neuroblastoma. Immunotherapy offers a novel approach to treating high-risk and relapsed disease, with the potential for durable responses and improved long-term outcomes.
Stem Cell Transplantation
Autologous stem cell transplantation is a critical component of treatment for high-risk neuroblastoma, allowing for the delivery of high-dose chemotherapy to eradicate cancer cells while preserving the patient’s hematopoietic stem cells. Stem cell transplantation enables the administration of intensive chemotherapy regimens that would otherwise be too toxic for the bone marrow. By replenishing the patient’s stem cells following myeloablative therapy, stem cell transplantation supports hematopoietic recovery and reduces the risk of treatment-related complications. Allogeneic stem cell transplantation may be considered in select cases to provide additional immune-mediated antitumor effects.
Multimodal Approaches
Disease management involves a coordinated mix of surgery, chemotherapy, radiation therapy, targeted therapies, and immunotherapy. Collaborative care teams create personalized treatment plans to optimize efficacy, minimize side effects, and enhance survival rates for pediatric patients.
Prognosis and Survival
Survival rates vary significantly depending on the risk group of the neuroblastoma:
- Low-risk group: 5-year survival rate higher than 95%.
- Intermediate-risk group: 5-year survival rate around 90-95%.
- High-risk group: 5-year survival rate around 50%.
- The 10-year overall survival rate for stage 1-3 neuroblastoma patients was approximately 91%, while for stage 4 cases older than 18 months, it was 38%.
- Surgery has been shown to significantly improve patient’s quality of life and prolong their survival.
- Other prognostic factors include age, with younger children generally having better outcomes, as well as tumor histology and stage at diagnosis.
- Recent improvements in medical care have led to better survival outcomes for neuroblastoma patients diagnosed in more recent years.
- Limitations of the data include lack of detailed treatment information and omission of certain molecular/genetic factors that can also influence prognosis.
- The Canadian Cancer Society reports the 5-year observed survival for neuroblastoma in children 0-14 years is 81%.
Challenges and Considerations
- High-Risk Disease Management: High-risk group requires multimodal therapy, including chemotherapy, surgery, radiotherapy, and biological, and immunological treatments. This aggressive approach aims to improve outcomes for patients with high-risk diseases.
- Prognostic Factors: Molecular markers like MYCN amplification play a crucial role in predicting prognosis. Patients with MYCN-amplified tumors, especially in stage 4 disease, have poorer survival rates. Other molecular tumor markers like 11q allelic status and tumor cell ploidy are also important prognostic factors.
- Age and Stage: Age at diagnosis and disease stage are significant prognostic indicators. Younger children, especially those under 18 months without MYCN amplification, have better survival rates. Accurate staging and risk stratification are essential for tailoring therapy and improving outcomes.
- Treatment Advances: Advances in treatment through clinical trials and research have led to improved outcomes for high-risk group patients. Current treatment strategies include intensive chemotherapy, radiation therapy, autologous stem cell transplant, and immunotherapy, with ongoing efforts to identify more targeted treatments and reduce toxicity.
These challenges and considerations highlight the complexity of managing disease, emphasizing the importance of personalized treatment approaches, understanding molecular markers, and advancing therapies to improve outcomes for patients with this aggressive pediatric cancer.

A 7-year-old girl who beat rare stage 4 cancer celebrates her remission by sharing touching photos of her first and last days of school
During Treatment
Common side effects include:
- Chemotherapy Side Effects: Chemotherapy can cause constipation, fatigue, hair loss, mouth sores, nausea, vomiting, and tingling or numbness in the hands and feet.
- Radiation Therapy Side Effects: Radiation therapy can cause skin irritation, fatigue, and changes in appetite and taste. It can also lead to long-term effects such as growth and development issues, thyroid problems, and fertility problems.
- Surgery Side Effects: Surgery can cause pain, swelling, and bruising at the site of the incision. It can also lead to long-term effects such as changes in the shape of the body, especially if the tumor is located near the spine or other bones.
- Immunotherapy Side Effects: Immunotherapy can cause flu-like symptoms, such as fever, chills, and fatigue. It can also lead to long-term effects such as changes in the immune system, which can increase the risk of infections.
- Other Side Effects: Other side effects of cancer treatment include changes in the way the body functions, such as changes in the thyroid, growth hormones, and bone growth. It can also cause changes in the reproductive organs, hearing, and lungs.
These side effects can vary depending on the type and dose of treatment, as well as the age of the child at the time of treatment. It is essential for children undergoing treatment to have regular check-ups with their healthcare provider to monitor for and manage these side effects effectively.
Management Strategies
A strategy to prevent the occurrence of side effects during disease treatment involves proactive management and monitoring throughout the treatment process. This includes:
- Patient Education: Providing comprehensive information to patients about potential side effects associated with their treatment, empowering them to recognize and report any symptoms promptly.
- Regular Monitoring: Conduct regular check-ups and assessments to monitor the patient’s response to treatment, detect early signs of side effects, and adjust the treatment plan accordingly.
- Individualized Treatment Plans: Tailoring treatment plans based on the patient’s risk factors, medical history, and specific needs to minimize the likelihood of adverse reactions.
- Supportive Care: Offering supportive care measures such as hydration, nutrition support, pain management, and psychological support to enhance the patient’s overall well-being and resilience during treatment.
- Collaborative Approach: Involving a multidisciplinary team of healthcare professionals, including oncologists, nurses, pharmacists, and supportive care specialists, to coordinate care and address side effects comprehensively.
By implementing these strategies, healthcare providers can proactively address potential side effects, optimize treatment outcomes, and improve the overall quality of care for patients undergoing neuroblastoma treatment.
After Treatment
Follow-up Care
- Close Monitoring: Follow-up visits are scheduled every 2-6 months for the first few years after treatment, then less often over time. These visits involve physical exams, imaging tests, and monitoring for any signs of recurrence or late effects 29.
- Screening for Late Effects: Patients undergo tests to check for potential long-term side effects, such as heart, lung, and hearing problems. This screening helps detect and manage any late effects early on.
- Transition to Adult Care: As patients grow up, they need to have their medical records and be able to communicate the details of their cancer diagnosis and treatment to new healthcare providers. Maintaining health insurance coverage is also crucial as patients transition to adult care.
- Recurrence Risk: The risk of disease recurrence is highest within the first 2 years after treatment, so close monitoring is essential during this period. However, late relapses can still occur, so ongoing follow-up is recommended.
- Supportive Care: Follow-up care involves a multidisciplinary team, including social workers and other professionals, to address the emotional, social, and practical needs of patients and their families.
- Long-Term Follow-Up Guidelines: The Children’s Oncology Group provides comprehensive long-term follow-up guidelines for childhood cancer survivors, which can help guide the care of patients.
Recommendations for Patients
- Regular Follow-Up Visits: Patients should attend follow-up visits every 2-6 months for the first few years after treatment, with less frequent visits over time. These visits involve physical exams, imaging tests, and monitoring for any signs of recurrence or late effects of treatment.
- Communication with the Healthcare Team: Patients should maintain open communication with their healthcare team, reporting any new symptoms or concerns promptly. It is essential to discuss any changes in health status with the medical team to address them effectively.
- Awareness of Late Effects: Patients should be aware of potential late effects of treatment, such as heart, lung, and hearing problems. Understanding these risks can help patients recognize and manage any long-term side effects that may arise.
By following these recommendations, neuroblastoma patients can actively participate in their post-treatment care, monitor their health effectively, and address any potential issues that may arise as they transition into survivorship.
Resources
- American Cancer Society – Cancer.org
- National Cancer Institute – Cancer.gov
- UChicago Medicine – Uchicagomedicine.org
- University of California San Francisco – Ucsfbenioffchildrens.org
- National Library of Medicine -Ncbi.nlm.nih.gov
- American Society of Clinical Oncology (ASCO) – Cancer.net
- Canadian Cancer Society – Cancer.ca/en
- Neuroblastoma UK – Neuroblastoma.org.uk
- Memorial Sloan Kettering Cancer Center – mskcc.org
- Children’s Hospital of Philadelphia – Chop.edu
- Oncodaily.com
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
