Nivolumab plus Cabozantinib Maintains Long-Term Survival Benefit in Advanced Renal Cell Carcinoma
Authors: T. Powles, M. Burotto, B. Escudier, A. B. Apolo, M. T. Bourlon, A. Y. Shah, C. Suárez, C. Porta, C. H. Barrios, M. Richardet, H. Gurney, E. R. Kessler, Y. Tomita, J. Bedke, S. George, C. Scheffold, P. Wang, V. Fedorov, R. J. Motzer, T. K. Choueiri
Published in ESMO Open on April 20, 2024
Introduction:
This study presents the extended follow-up results from the phase III CheckMate 9ER trial, evaluating the efficacy and safety of the combination of nivolumab (an immune checkpoint inhibitor) and cabozantinib (a tyrosine kinase inhibitor) versus sunitinib in previously untreated advanced or metastatic renal cell carcinoma (RCC).
Design and Methods:
The open-label, randomised phase III trial enrolled 651 patients with treatment-naïve advanced or metastatic RCC. Patients were randomly assigned 1:1 to receive either nivolumab plus cabozantinib or sunitinib. The primary endpoint was progression-free survival (PFS), and secondary endpoints included overall survival (OS), objective response rate (ORR), and safety.
What We Learned:
After a median follow-up of 44.0 months, the nivolumab plus cabozantinib combination continued to demonstrate superior efficacy over sunitinib:
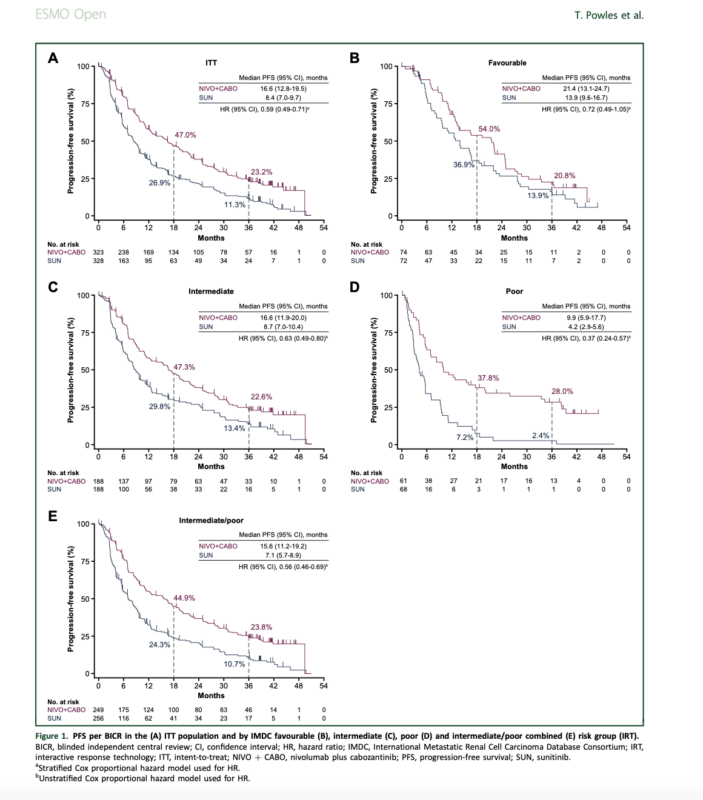
- Median PFS was 16.6 months with nivolumab plus cabozantinib versus 8.4 months with sunitinib (hazard ratio [HR] 0.59).
- Median OS was 49.5 months with nivolumab plus cabozantinib versus 35.5 months with sunitinib (HR 0.70).
- ORR was 56% with nivolumab plus cabozantinib versus 28% with sunitinib, with higher complete response rates (13.3% vs. 4.9%).
Key Highlights:
- The survival and response benefits were maintained across intermediate, poor, and intermediate/poor International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk groups.
- Patients who completed the planned two years of nivolumab treatment had a median time to subsequent therapy of 20.6 months, suggesting durable efficacy.
- The safety profile remained consistent with previous follow-ups, with no new safety signals emerging.
- Treatment-related adverse events occurred in 97.2% (any grade) and 66.9% (grade ≥3) of patients in the nivolumab plus cabozantinib arm, compared to 93.1% (any grade) and 55.3% (grade ≥3) in the sunitinib arm.
Key Takeaway Messages:
- The extended follow-up data from the CheckMate 9ER trial continues to support the use of nivolumab plus cabozantinib as a first-line treatment option for advanced or metastatic renal cell carcinoma, offering improved progression-free survival, overall survival, and response rates compared to sunitinib.
- The survival and response benefits were observed across various IMDC risk groups, particularly in the intermediate, poor, and intermediate/poor combined subgroups.
- The safety profile remained consistent with previous follow-ups, and no new safety signals emerged with longer follow-ups.
- These results reinforce the potential for durable clinical benefit with the nivolumab plus cabozantinib combination in the first-line setting for advanced or metastatic renal cell carcinoma.
Summary by Amalya Sargsyan, MD
About OncoDaily
OncoDaily was founded in 2023. It is a US-based oncology media platform, which features the latest news, insights, and patient stories from the world of oncology. Within a short period of time it became one of the leading oncology media platforms globally.
OncoDaily gathers content from various sources, including social media posts from renowned oncologists from all over the world, news from oncology societies and cancer centers, patient and survivor stories, and career-related information for professionals.
The mission of OncoDaily is to empower patients, survivors, and professionals with the knowledge and inspiration they need to fight cancer. The motto of OncoDaily is “Cancer doesn’t take a day off – neither do we”.
