Piotr Wysocki, Professor of Medicine and Head of the Department of Oncology at Jagiellonian University Hospital, shared on LinkedIn:
“The final results of a CLEAR study have been published in print in the Journal of Clinical Oncology. This study randomized 1069 advanced clear-cell, renal-cell cancer (ccRCC) patients in a 1:1:1 ratio to receive lenvatinib and pembrolizumab, lenvatinib and everolimus or sunitinib. An interim analysis published in 2021 (NEJM 2021;384:1289-1300) demonstrated significant improvement of PFS (primary endpoint) with lenvatinib+pembrolizumab compared to sunitinib (no such effect was shown for lenvatinib+everolimus).
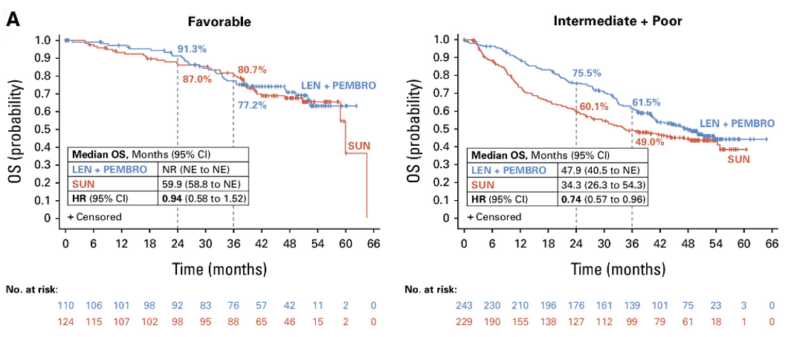
In the current publication, the authors provided data on the final prespecified OS analysis comparing lenvatinib+pembrolizumab with sunitinib. At the median OS follow-up time of 49.8 months, the median OS was 53.7 months and 54.3 months in the lenvatinib plus pembrolizumab and sunitinib groups, respectively. This translated into a 21%, marginally significant reduction in the risk of death with lenvatinib+pembrolizumab compared to sunitinib (HR for OS – 0.79; 95% CI, 0.63 to 0.99). ccRCC patients in intermediate and poor risk groups derived significant benefit from the lenvatinib+pembrolizumab combination in terms of OS (HR for OS = 0.74; 95%CI 0.57-0.96) while there was no difference between study arms in terms of overall survival in favorable-risk patients (HR for OS = 0.94; 95%CI 0.58-1.52).
In summary – the CLEAR study results agree with other, similarly constructed studies – CheckMate 9ER, Keynote 426. All these studies demonstrated that such aggressive (immunotherapy+TKI) combinations should be offered in intermediate and poor-risk ccRCC patients where, in addition to a high and fast overall response rate, they significantly improve both PFS and OS. However, the use of immunotherapy-TKI combinations should not be considered a standard approach to favorable-risk patients (usually asymptomatic or mild-symptomatic patients) in whom a single-agent sunitinib-based therapy demonstrates a much more convenient safety profile, enables sequential single-agent treatment, and first-of-all does not negatively impact overall survival.”
Source: Piotr Wysocki/LinkedIn
