Mehdi Hamadani, Director of the Bone Marrow Transplantation and Cellular Therapy Program at the Medical College of Wisconsin, shared a post on X/Twitter:
“Much awaited Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1506 trial out in the Journal of Clinical Oncology establishing gilteritinib as SOC post-allo-bone marrow transplant maintenance in minimal residual disease (MRD) ve FLT3-ITD AML patients in CR1; thanks to a global collaboration funded by Astellas Pharma, the National Institutes of Health (NIH) and the National Cancer Institute (NCI).
Before getting an industry trial label, remember this trial was conceived as #1 idea in the 2014 BMTCTN State of the Science Symposium white paper thinks to the visionary leadership of Steven Devine, F. Appelbaum, Rick Jones, Martin S. Tallman et al. in Leukemia committee.
Thankful to my mentor Mary Horowitz for trusting me as protocol officer for this mammoth study. The first email about the trial in folder is from 3/18/2015. 9 years of incredible collaboration across 16 countries, 122 centers and 600+ patients screened. Kudos to cochair YB Chen and Mark Levis.
Many questioned the ethics of this trial after Sormain results, but we discussed the ethic of protocol in a 2019 Journal of Clinical Oncology position statement. Thank you to Charlie Craddock for publishing that editorial that proved vital for completion of this study.
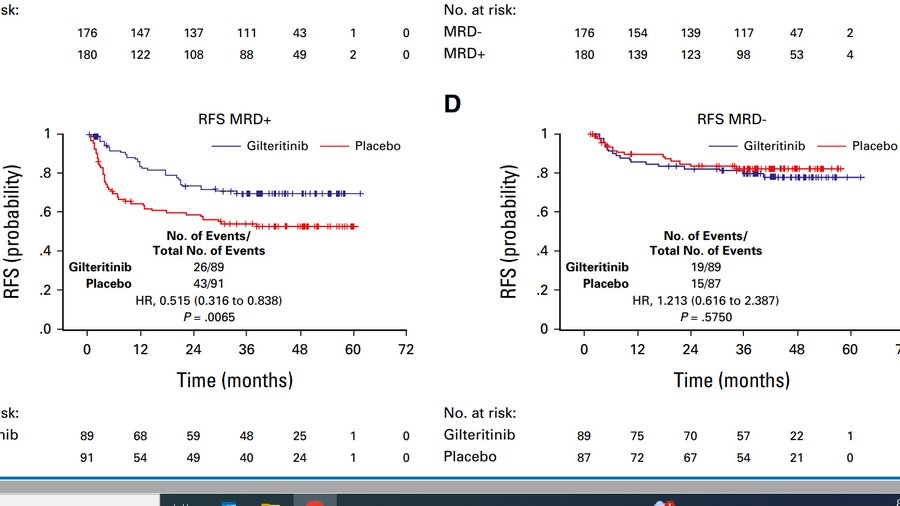
Study randomized 356 FLT3-ITD+ patients in CR1 to gilterinib vs. placebo stratified on conditioning intensity; time post HCT and MOST IMPORTANTLY MRD status. We suspected that the benefit of maintenance will be restricted to MRD+ patients.
In overall population RFS just missed the mark (HR, 0.679; two-sided P = .0518). Trial required 122 events for 85% power. We had to stop follow up at 103 events, because event rate was so slow that 122 events would have need another 10 years. This was really underpowered!
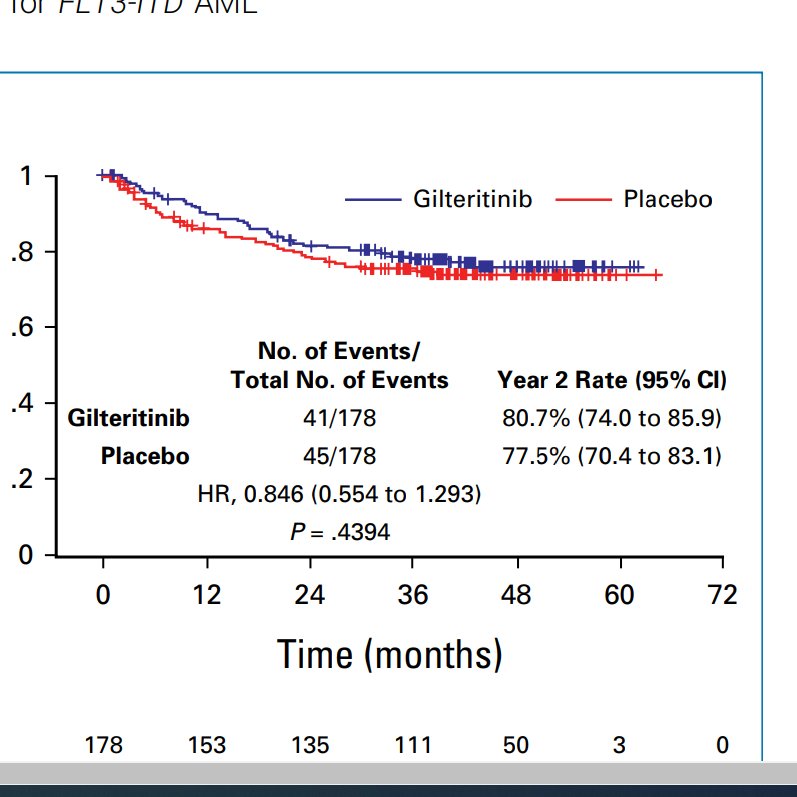
Also look at modern alloHCT outcomes across 16 countries. Regardless of arm, 2 year OS curves below are stellar. ~80% OS at two years; just wow.
The key finding of the trial is; if you have access to a proper MRD assay (10^6) save MRD- patients toxicity of maintenance, but judging by curves below, not providing maintenance to an MRD+ patient can no longer be considered evidence based care!
Oh yes, simple FLT3 diagnostic tests are NOT MRD assays. MCW Cancer Center we use the invivoscribe MRD testing assay (similar what BMTCTN trial used).
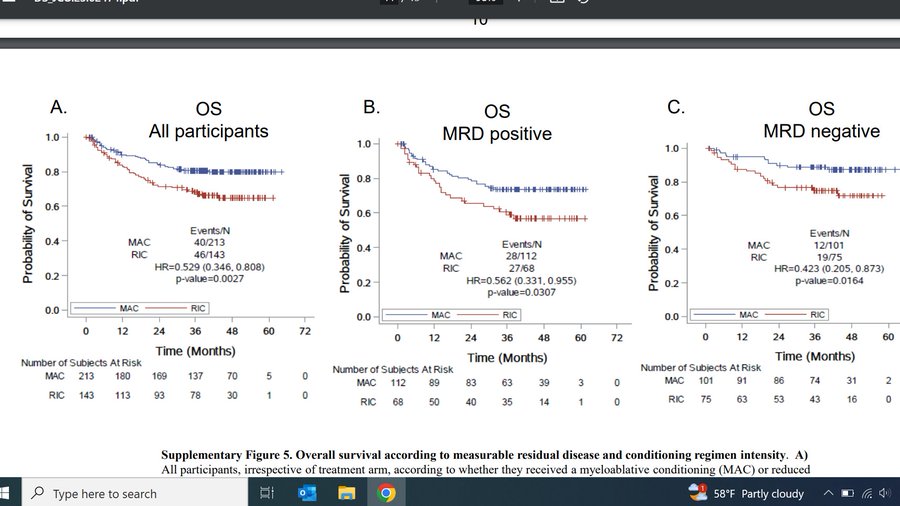
Hidden in supplement is a practice nugget. We again show, when safe conditioning intensity in AML matters. MAC patients had OS benefit regardless of MRD status!
Worth reading editorial on 1506 by Charlie Craddock and Naval Daver Moving Toward Total Therapy in AML: Personalized Treatments Improve Post-Transplant Outcome | Journal of Clinical Oncology.”
Source: Mehdi Hamadani/X