George Mellgard, Medical Student at Columbia University Irving Medical Center in NYC, shared on X/Twitter:
“With the ongoing conversation on the FDA Accelerated Approval process – I am excited to share our work!! We delve into the complexities of FDA accelerated approvals in oncology using lessons from 16 withdrawn AAs in the past decade (2012 – 2022). Lesson to follow!

Failure to confirm approval data -> in approval trials increases rates of discontinuation in control (olaratumab) or treatment arms (atezolizumab) inflated initial results and was not replicated with confirmatory trials.
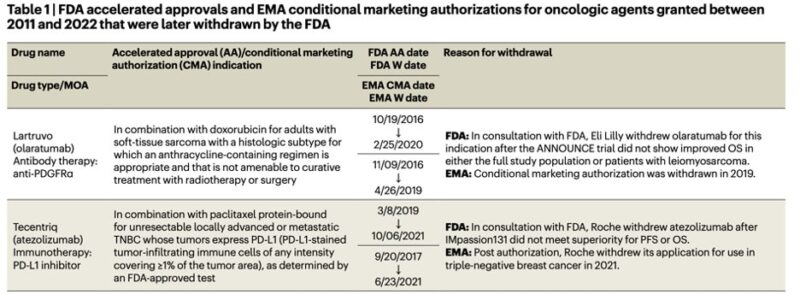
Failure against standard of care -> ICI approval trials relied on increasing ORR with monotherapy and failed to be replicated against chemotherapy. “Crossing of the survival curves” (e.g. DANUBE) is understood as a lack of chemotherapy in ICI non-responders.
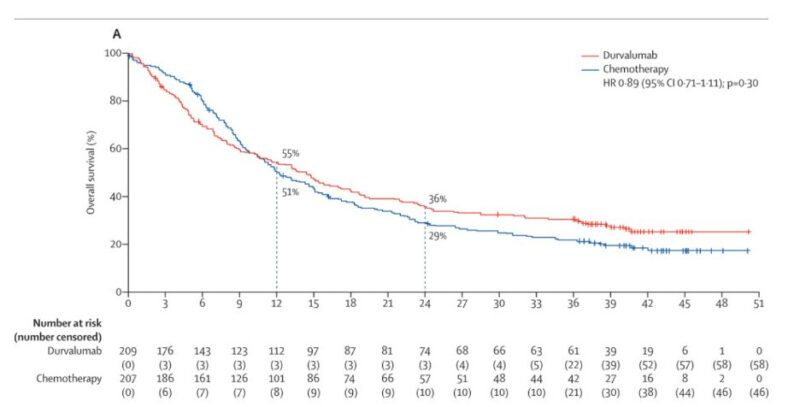
Failure due to trial design -> in brief Romidepsin and VSLI’s AAs were withdrawn due to flawed post-marketing trials aiming to broaden their initial limited uses into earlier lines of treatment.

Changes in the risk-benefit equation -> For PI3Ki AAs, increased Grade 3 and 4 AEs in approval trials highlight how toxicities should alter the assessment of clinical benefit using surrogate endpoints.
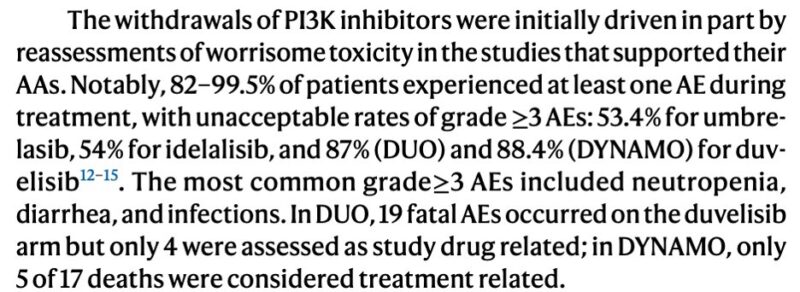
Ultimately withdrawals provide a diverse portrait of drug development. We affirm ongoing support for FDA AAs in oncology and conclude we should not let “the best be the enemy of the good.” Grateful to mentors, Drs. Bates and Fojo for their guidance.”
Source: George Mellgard/X
