
Olomorasib Receives FDA Breakthrough Therapy Designation for KRAS G12C-Mutant NSCLC
On September 4, 2025, Eli Lilly and Company announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation to olomorasib (LY3537982) in combination with pembrolizumab (KEYTRUDA) as a potential first-line treatment for patients with unresectable or metastatic non-small cell lung cancer (NSCLC) harboring a KRAS G12C mutation and PD-L1 expression ≥50%, as confirmed by FDA-approved tests.
‘‘The Breakthrough Therapy designation recognizes the potential for olomorasib to be a meaningful treatment advance and highlights the continued unmet need for improved options for patients with KRAS G12C-mutant NSCLC, particularly in the first-line setting in combination with standard-of-care immunotherapy”
said David Hyman, M.D., Chief Medical Officer, Lilly.

LinkedIn photo of David Hyman
What is Olomorasib?
Olomorasib, developed by Lilly, is a potent, selective, small-molecule inhibitor of the KRAS G12C oncoprotein, designed for the treatment of advanced solid tumors harboring this mutation, including non-small cell lung cancer (NSCLC). KRAS G12C drives constitutive signaling that promotes uncontrolled tumor growth. Olomorasib binds covalently to the mutant KRAS G12C protein, locking it in an inactive GDP-bound state and inhibiting downstream oncogenic signaling pathways.
Its targeted mechanism allows for selective tumor inhibition while minimizing effects on normal cells. In clinical studies, Olomorasib is being evaluated both as monotherapy and in combination with chemoimmunotherapy or immunotherapy to enhance antitumor activity and optimize clinical outcomes.
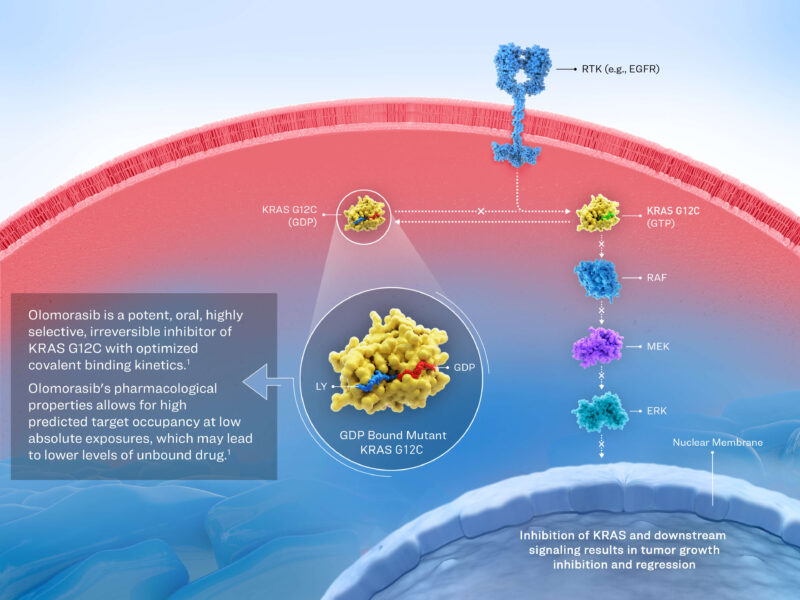
source: Lilly Official Website
What research supports the designation?
The FDA granted Breakthrough Therapy designation to olomorasib based on promising data from the Phase 1/2 LOXO-RAS-20001 trial and the dose optimization phase of the Phase 3 SUNRAY-01 study. Updated results from these trials will be presented at the 2025 World Conference on Lung Cancer (WCLC) in Barcelona, September 6–9.
Olomorasib + Pembrolizumab in 1L KRAS G12C NSCLC: Integrated Results
Prior results from an integrated analysis show that olomorasib, a selective second-generation KRAS G12C inhibitor, has demonstrated encouraging antitumor activity and a manageable safety profile when combined with pembrolizumab. Among 85 patients—63% with PD-L1 ≥50% and 17% having received one prior pembrolizumab cycle—treatment with olomorasib (50 or 100 mg BID) plus pembrolizumab (200 mg Q3W) resulted in common any-grade adverse events including diarrhea (29%) and ALT/AST elevations (26%/25%), with grade ≥3 events primarily ALT/AST increases (18%/14%) and diarrhea (7%).
Dose reductions were required in 29% of patients, and 9% discontinued treatment due to adverse events. The overall response rate (ORR) was 71% across all patients and 85% in PD-L1 ≥50% patients receiving 100 mg BID. Responses were durable, with median duration of response not yet reached and a 6-month progression-free survival of 77%. These prior results support continued investigation in the Phase 3 SUNRAY-01 trial comparing olomorasib 100 mg BID plus pembrolizumab versus placebo plus pembrolizumab as first-line therapy in KRAS G12C–mutant NSCLC with PD-L1 ≥50%. (Abstract #MA02.06).
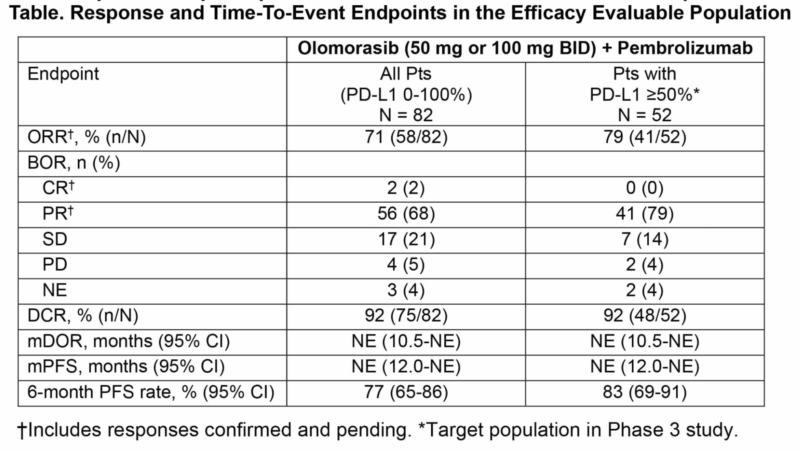
Olomorasib + Chemoimmunotherapy in 1L KRAS G12C NSCLC: Integrated Results
Prior results from an integrated analysis show promising antitumor activity and a manageable safety profile with chemoimmunotherapy. Among 78 patients—35% PD-L1 <1%, 40% PD-L1 1–49%, 22% PD-L1 ≥50%, and 31% having received one prior SOC cycle—common any-grade TRAEs included nausea (37%), anemia (35%), fatigue (32%), diarrhea (30%), and AST/ALT increases (27%/26%). Grade ≥3 TRAEs were primarily anemia (14%), neutrophil count decrease (12%), and ALT/AST elevation (12% each).
Dose reductions occurred in 15% and treatment discontinuation in 6%. Overall response rate (ORR) was 59% overall and 64% in patients receiving 100 mg BID. Responses were durable, with median DOR of 10.5 months and median PFS of 11.6 months. These prior results support the ongoing Phase 3 SUNRAY-01 trial comparing olomorasib 100 mg BID plus chemoimmunotherapy versus placebo plus chemoimmunotherapy as first-line therapy in KRAS G12C–mutant NSCLC. (Abstract #OA08.02)
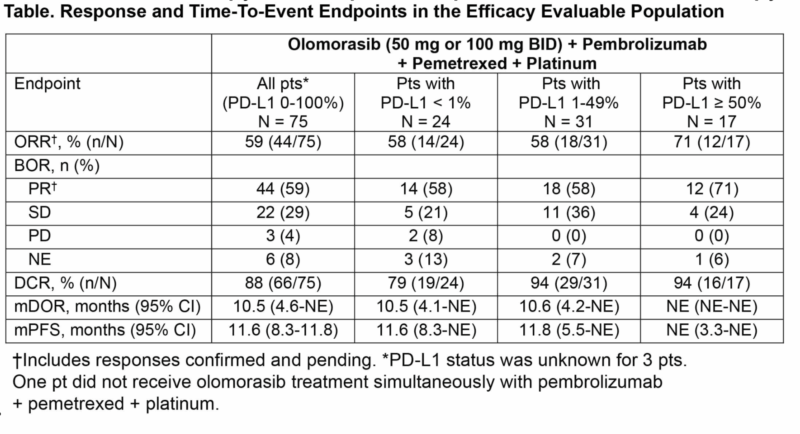
The submitted abstracts for both presentations were based on data up to January 15, 2025, while the upcoming oral presentations will include updated data through June 6, 2025.
LOXO-RAS-20001 trial
The LOXO-RAS-20001 trial (NCT04956640) is an open-label, multicenter Phase 1/2 study evaluating olomorasib, a selective KRAS G12C inhibitor, in patients with advanced solid tumors harboring KRAS G12C mutations, including non-small cell lung cancer (NSCLC).
- Phase 1a (dose escalation): Explored the safety, tolerability, pharmacokinetics, and recommended dose of olomorasib monotherapy in KRAS G12C–mutant tumors.
- Phase 1b (dose expansion/optimization): Evaluated olomorasib both as monotherapy and in combination with other agents, including pembrolizumab and chemotherapy, to optimize dosing and assess early efficacy.
- Phase 2: Focused on expanded patient cohorts to further evaluate efficacy and confirm the recommended Phase 3 dose in NSCLC and other solid tumors.
The primary endpoints included safety and tolerability, determination of the maximum tolerated dose and recommended Phase 2 dose, and objective response rate per RECIST v1.1. Secondary endpoints included duration of response, progression-free survival, overall survival, pharmacokinetics, and exploratory biomarker analyses.
Results
LOXO-RAS-20001 trial’s results, published in the Journal of Clinical Oncology (Volume 43, 2025; Abstract 8519, 2025 ASCO Annual Meeting), included 43 patients with a median age of 70. Among them, 44% had PD-L1 ≥50%, 30% had PD-L1 1–49%, 23% were PD-L1 negative, and 2% were unknown. At a median follow-up of 5.5 months, treatment-related adverse events were manageable, with the most common being elevated liver enzymes (ALT 33%, AST 30%), diarrhea (28%), fatigue (16%), and nausea (14%). Grade 3 liver enzyme elevations occurred in 26% (ALT) and 16% (AST), controlled through dose adjustments or corticosteroids. Pneumonitis occurred in two patients. Dose reductions were required in 16% and 5% discontinued treatment due to adverse events.
Among 40 efficacy-evaluable first-line patients, the objective response rate (ORR) was 70%, including one complete and 23 partial responses, with a disease control rate (DCR) of 90%. In patients with PD-L1 ≥50%, ORR reached 82% and DCR 94%. Median duration of response was not yet reached, and the six-month progression-free survival rate was 80%.

Read more about Advances in KRAS G12C-Mutated NSCLC: Key Insights from ELCC 2025 on OncoDaily.
SUNRAY-01 trial
SUNRAY-01, a global, randomized, double-blind, placebo-controlled Phase 3 study, is investigating olomorasib plus pembrolizumab, with or without chemotherapy, versus placebo plus standard-of-care regimens in treatment-naïve patients with KRAS G12C–mutant metastatic NSCLC. The primary endpoint is progression-free survival, with secondary endpoints including overall survival, response rates, and safety outcomes. Early findings from the dose optimization phase indicated that the addition of olomorasib may improve clinical benefit without introducing prohibitive toxicity.
Ongoing trials with Olomorasib
Among its ongoing studies, Eli Lilly is conducting a Phase 1 pharmacokinetic trial in healthy participants (NCT07044271). This open-label, randomized crossover study evaluates how Olomorasib is absorbed, distributed, and cleared after multiple oral doses. The trial lasts about seven weeks, including a series of inpatient stays for detailed monitoring.
Find more interviews on OncoDaily TV.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
