CAR T-Cell Therapy vs iNKT Cell Therapy: The Future of Engineered Immune Cells in Cancer Treatment
The field of adoptive cell therapy has undergone a dramatic transformation over the past two decades. What began with early experiments in T-cell receptor engineering has matured into a rapidly expanding clinical frontier, marked by the advent of chimeric antigen receptor (CAR) T-cell therapy. In 2017, the U.S. FDA approved the first CAR T-cell product for pediatric acute lymphoblastic leukemia (ALL), marking a paradigm shift in oncology (Maude et al., 2018). Yet, as promising as these autologous therapies have been, their logistical complexity, toxicity, and cost have prompted researchers to explore off-the-shelf immune cell alternatives—notably, invariant natural killer T (iNKT) cells.
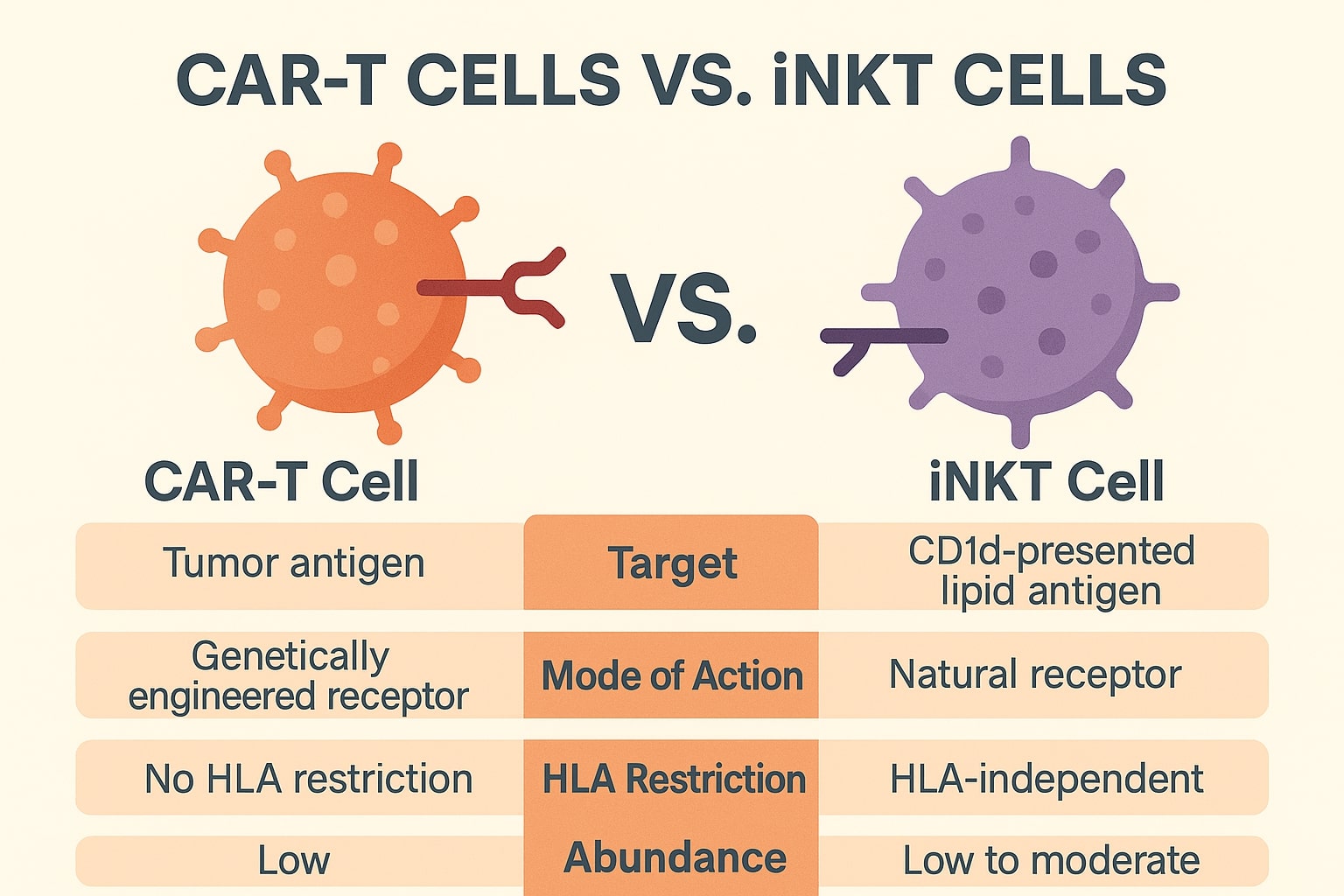
In the evolving landscape of CAR T-cell therapy vs iNKT cell therapy, iNKT cells represent a rare, innate-like T-cell population that bridges the gap between innate and adaptive immunity. Unlike CAR T-cells, which must be harvested and engineered from each individual patient, iNKT cells offer a universal, allogeneic platform. With advances in induced pluripotent stem cell (iPSC) technology and gene editing, iNKT-based therapies are now entering the clinical stage with the hope of solving some of CAR T’s most intractable challenges (Zhao et al., 2020).
CAR T-Cells: Personalized Powerhouses Against Blood Cancers
Chimeric Antigen Receptor T-cell (CAR T-cell) therapy has transformed the treatment landscape for several hematologic malignancies, offering durable remissions in diseases previously considered incurable. Unlike conventional therapies, CAR T-cells are personalized immune cells engineered to recognize and kill cancer cells with exceptional specificity and potency.
The breakthrough came with tisagenlecleucel (Kymriah), the first FDA-approved CAR T-cell therapy in 2017 for pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL). The ELIANA trial demonstrated a remarkable overall remission rate of 81% within three months of infusion (Maude et al., 2018). Following this success, axicabtagene ciloleucel (Yescarta) was approved for diffuse large B-cell lymphoma (DLBCL), supported by the ZUMA-1 trial, which showed complete response (CR) rates around 58% (Locke et al., 2019).
Today, CAR T-cells are approved for multiple indications, including:
- B-cell acute lymphoblastic leukemia (ALL) in children and young adults
- Relapsed/refractory large B-cell lymphoma, including DLBCL, high-grade B-cell lymphoma, and primary mediastinal B-cell lymphoma
- Mantle cell lymphoma
- Multiple myeloma, with idecabtagene vicleucel (Abecma) and ciltacabtagene autoleucel (Carvykti) targeting BCMA
CAR T-cell therapy works by isolating a patient’s own T-cells, modifying them ex vivo to express a chimeric receptor that binds a tumor-associated antigen (e.g., CD19 or BCMA), expanding them in the lab, and reinfusing them to attack cancer cells. This highly targeted approach not only spares healthy tissues but also leads to memory T-cell formation, offering potential for long-term surveillance against relapse.
Despite high response rates, not all patients achieve durable remission. Relapse can occur due to antigen loss, T-cell exhaustion, or inadequate persistence of CAR T-cells. However, efforts are underway to develop next-generation CAR designs—including dual-targeting CARs, co-stimulatory domain enhancements, and armored CARs—to improve outcomes and prevent immune escape (Rafiq et al., 2020).
In short, CAR T-cell therapy represents a paradigm shift in hematologic oncology. For many patients with limited options, it offers renewed hope and, in some cases, a second chance at life.

Read About CAR T-Cell Therapy on OncoDaily
iNKT Cells: The Rise of Off-the-Shelf Immunotherapy
Invariant Natural Killer T (iNKT) cells represent a novel frontier in cell-based cancer therapy—offering the promise of “off-the-shelf” immunotherapy with broad applicability and reduced risk of adverse immune reactions. Unlike CAR T-cells, which are tailored to each patient, iNKT therapies can be mass-produced from allogeneic sources, including induced pluripotent stem cells (iPSCs), making them scalable, accessible, and cost-effective.
iNKT cells are a unique subset of T lymphocytes that combine features of both T cells and NK cells, enabling them to recognize lipid antigens presented by CD1d molecules—a distinct mechanism compared to MHC-restricted recognition. This allows iNKT cells to target a broader range of tumors, including those with low or absent MHC expression, which are typically resistant to conventional T-cell therapies (Gumperz et al., 2002).
One of the leading platforms in this space comes from Appia Bio, which is developing iNKT cells from hematopoietic stem cells using their ACUA™ platform. Another example is Acepodia, which is investigating antibody-conjugated iNKT cells designed to bind solid tumor antigens with the help of tumor-targeting antibodies. These advances offer a modular and rapid approach to creating iNKT products tailored for specific cancers.
The key advantage of iNKT cells lies in their inherent resistance to graft-versus-host disease (GvHD). Because they do not mediate strong alloreactivity, iNKT therapies can be safely administered across HLA barriers, removing the logistical barriers associated with autologous CAR T-cell manufacturing. Additionally, iNKT cells possess natural tumor-homing abilities and secrete Th1 cytokines such as IFN-γ and TNF-α, contributing to potent anti-tumor immunity (Song et al., 2009).
Preclinical studies and early-phase clinical trials have shown encouraging signs of activity in both hematologic malignancies and solid tumors. For instance, a Phase I study from MSKCC using CD19-CAR iNKT cells in lymphoma demonstrated tumor reduction and immune persistence without significant toxicity (Heczey et al., 2020). Researchers are also exploring dual-function iNKT cells capable of acting as both direct tumor killers and immune modulators, further enhancing their therapeutic scope.
Importantly, iNKT therapies lend themselves to uniform product quality and reduced time-to-treatment, potentially enabling earlier intervention in aggressive cancers. They may also reduce costs significantly, since centralized manufacturing circumvents the need for individualized processing and cold-chain logistics.
As the field advances, iNKT cell therapies are emerging not merely as alternatives to CAR T-cells, but as a complementary or even synergistic approach to reprogram the immune system against cancer.
Read About iNKT Cell Therapy on OncoDaily
Head-to-Head: Comparing Efficacy in Clinical Trials
Although CAR T-cells have already demonstrated impressive clinical activity in B-cell malignancies, data for iNKT therapies—especially CAR-engineered iNKT cells—are emerging from earlier-phase trials. That said, comparative insights are beginning to crystallize.
In large multicenter trials, such as ELIANA (CD19+ pediatric ALL), ZUMA-1 (DLBCL), and KarMMa (multiple myeloma), CAR T-cells have consistently shown overall response rates (ORR) exceeding 70%, with complete responses (CR) in up to 40–60% of cases depending on indication and product (Maude et al., 2018; Neelapu et al., 2017; Munshi et al., 2021). Durable remissions are possible but often limited by antigen escape and relapses.
By contrast, data on CAR-iNKT therapies is limited but promising. A first-in-human study of CD19-directed CAR-iNKT cells in patients with B-cell lymphoma demonstrated responses with no detectable GvHD, and in some cases better tissue infiltration and persistence than conventional CAR T-cells (Heczey et al., 2020). Early-stage trials of iPSC-derived iNKT cells have shown effective engraftment and cytotoxicity against CD1d+ leukemias (Watarai et al., 2022), although head-to-head comparisons remain pending.
Importantly, iNKT cells may provide greater safety, off-the-shelf availability, and enhanced tumor microenvironment modulation—features that give them a potential edge in scalability and broader application.
Toxicity Profiles: How Safe Are These Cell Therapies?
CAR T-cell therapy’s biggest success story is also its Achilles’ heel—potent immune activation. Nearly all patients experience cytokine release syndrome (CRS) to varying degrees, with rates of Grade ≥3 CRS in 13–22% of patients depending on the product (Neelapu et al., 2017; Lee et al., 2019). In addition, immune effector cell–associated neurotoxicity syndrome (ICANS), often manifesting as delirium, seizures, or cerebral edema, occurs in up to 30% of recipients (Gust et al., 2017).
iNKT cells—engineered or unmodified—appear to offer a safer toxicity profile. Because of their unique cytokine secretion patterns and reduced expansion in vivo, iNKT-based therapies have shown minimal CRS and negligible ICANS in both preclinical and limited human studies (Heczey et al., 2020). Moreover, their intrinsic immunoregulatory role and MHC independence greatly reduce the risk of GvHD, enabling allogeneic administration without intensive T-cell depletion protocols.
While more data are needed from later-phase trials, the lower immunotoxicity of iNKT therapies may position them as safer alternatives, especially for frail or elderly patients.
The Solid Tumor Challenge: Can Either Therapy Break Through?
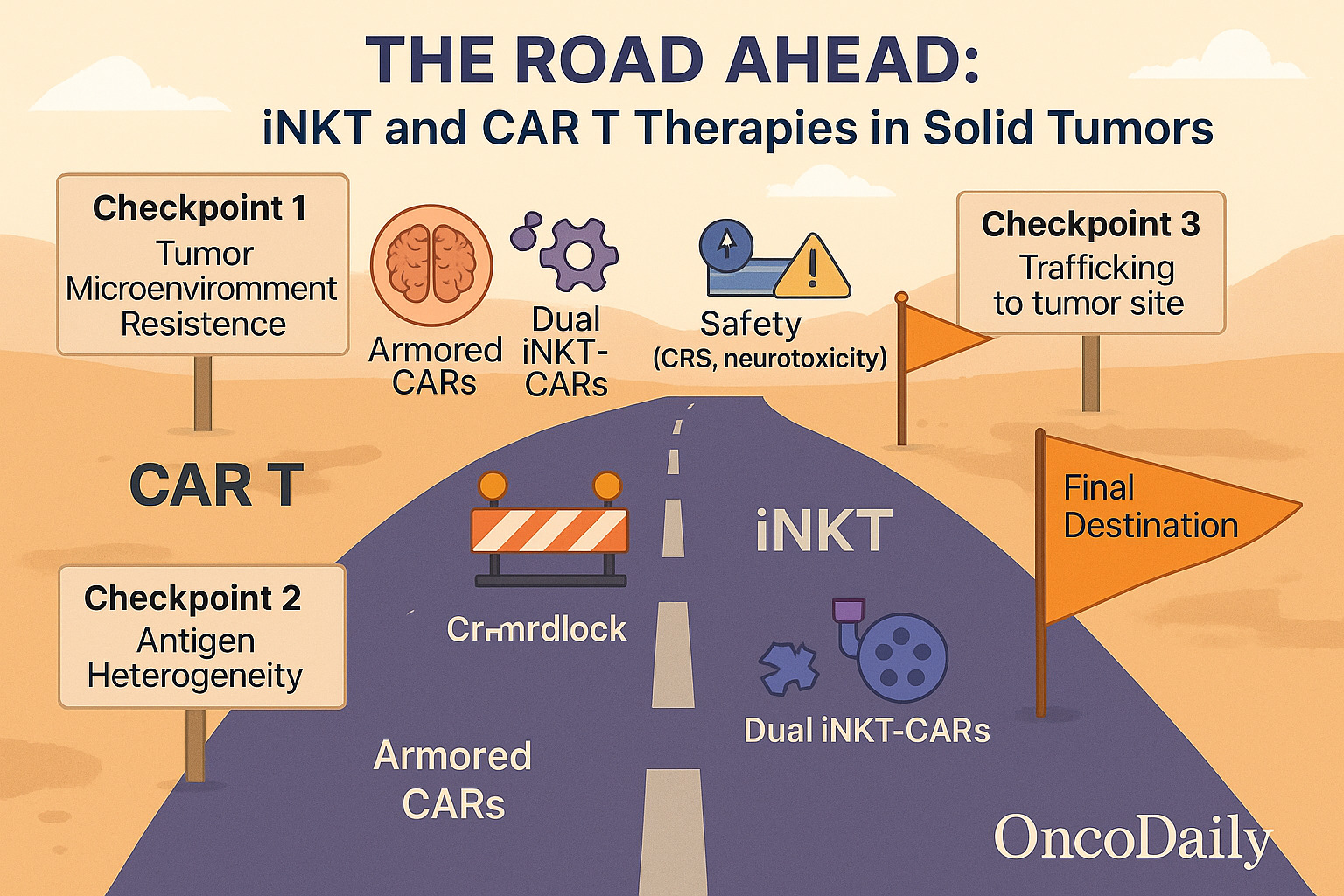
Despite their success in hematologic malignancies, solid tumors remain a major hurdle for both CAR T and iNKT therapies. CAR T-cells struggle with antigen heterogeneity, limited trafficking, and an immunosuppressive tumor microenvironment (TME). While various approaches—like dual CARs, armored CARs, and regional delivery—are being tested, no CAR T-cell has yet achieved FDA approval for solid tumors.
iNKT cells may be better suited for solid tumor infiltration due to their natural expression of chemokine receptors and their ability to kill CD1d+ tumor-associated macrophages and suppressive myeloid cells, thereby reshaping the TME (Berzofsky et al., 2021). CAR-iNKT constructs targeting GD2 in neuroblastoma and mesothelin in mesothelioma are in early clinical stages (Zhao et al., 2020), showing preclinical success in improving tumor penetration and cytotoxicity.
Nevertheless, both approaches face the central challenge of overcoming physical, immunologic, and metabolic barriers within solid tumors. Combinatorial strategies—such as checkpoint inhibitors, oncolytic viruses, and regional delivery—may be essential to unlock their full potential.
Manufacturing Matters: Speed, Cost, and Scalability
Autologous CAR T-cell manufacturing is expensive and time-consuming. From leukapheresis to infusion, the process can take 3–4 weeks, and some patients may deteriorate before receiving therapy. Additionally, failure rates in manufacturing range from 5–10% in real-world settings (Frey et al., 2021).
iNKT cells, particularly when derived from iPSCs or cord blood, can be manufactured at scale, cryopreserved, and stored as “off-the-shelf” therapies. This removes the need for individualized production and offers consistent product quality. Moreover, their reduced GvHD risk supports broader allogeneic use, potentially reducing costs and expanding access.
Scalable iNKT platforms are now being developed by biotech companies like Appia Bio, Nkarta, and Athenex, many in collaboration with academic institutions. The emergence of these platforms may signal a shift toward mass-produced immune cell therapies, not unlike monoclonal antibodies.
Regulatory Hurdles and Clinical Pipelines
As of 2025, six CAR T-cell products have received FDA approval for blood cancers, including Kymriah®, Yescarta®, Tecartus®, Breyanzi®, Abecma®, and Carvykti®. Each has undergone rigorous Phase I–III trials, often leading to breakthrough therapy designation based on durable responses in relapsed or refractory populations.
In contrast, iNKT-based therapies remain investigational. Ongoing trials include:
- NCT03774654: CD19-CAR-iNKT in B-cell malignancies
- NCT05894851: Allo-819 iPSC-iNKT in hematologic cancers
- NCT04825090: Mesothelin-targeting CAR-iNKT for mesothelioma
While regulatory progress is slower, the iNKT pipeline is rapidly expanding, especially with iPSC-derived platforms now demonstrating clinical-grade consistency and persistence.
Synergy, Not Rivalry: When CAR and iNKT Therapies Might Work Together
Rather than viewing CAR T and iNKT therapies as competitors, a synergistic approach may offer the best of both worlds. One proposed strategy involves combining CAR T-cells with iNKT infusions to reduce toxicities and enhance anti-tumor responses. Another possibility is using iNKT cells as a pre-conditioning regimen to modulate the immune microenvironment before CAR T administration.
Moreover, co-engineered dual-effector platforms, such as CAR T/iNKT hybrids or sequential cell therapy cocktails, are under development. In one study, CAR-iNKT cells provided both direct tumor cytotoxicity and TME modulation, enabling better CAR T-cell persistence (Zhao et al., 2020).
These hybrid strategies could become a cornerstone of next-generation cellular immunotherapy—multifaceted, layered, and patient-tailored.
What Lies Ahead: The Future of Cell-Based Cancer Therapy
As we enter a new era in oncology, both CAR T-cell and iNKT cell therapies are poised to reshape the therapeutic landscape. CAR T-cell therapy has already redefined outcomes in hematologic malignancies, with FDA-approved products demonstrating durable remissions in diseases like acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), and multiple myeloma. However, logistical constraints, toxicity profiles, and high costs limit their scalability and accessibility—especially in low-resource settings.
In contrast, iNKT cell therapy, propelled by recent 2025 clinical data, offers a highly attractive off-the-shelf immunotherapy platform. Their innate ability to avoid graft-versus-host disease (GVHD), resist exhaustion, and modulate the tumor microenvironment makes them uniquely suited for broader applications, including cell therapy for solid tumors, where CAR T-cells continue to struggle. The universal donor potential of iNKT cells—combined with promising early trial results in neuroblastoma, prostate cancer, and germ cell tumors—positions them as a complementary or alternative strategy to autologous CAR T-cell approaches.
Importantly, the future of engineered immune cell therapy may not be binary. Emerging translational work suggests synergistic potential in CAR T-cell therapy vs iNKT cell therapy: using iNKT cells to precondition the immune environment or enhance CAR T-cell function through cytokine support or immune remodeling. Dual-targeting constructs, armored iNKTs, and combination regimens are already being investigated in early-phase trials.
Ultimately, cell-based immunotherapy is shifting from a single-modality intervention toward a diversified, layered strategy. As platforms mature and comparative data grow, clinicians and researchers must adopt a disease- and patient-specific mindset, choosing the right cell for the right setting. The next frontier lies not only in refining these therapies but in making them faster, safer, and more universally accessible.
Read More About Immunotherapy Novels on Our OncoDaily IO page
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
