Invariant natural killer T (iNKT) cells represent a distinct lymphocyte subset characterized by a semi-invariant T-cell receptor that recognizes lipid antigens, such as α-galactosylceramide (α-GalCer), presented via CD1d molecules (Traversing bench to bedside: Clinical translation of iNKT cell therapy, 2024). While numerically scarce in peripheral blood, iNKTs are uniquely positioned to instigate potent and rapid immune responses. Upon encountering antigen, they secrete a broad spectrum of cytokines—including interferon-gamma (IFN-γ) and interleukin-4 (IL-4)—that quickly activate multiple components of both innate and adaptive immunity (Yin-Ruide, Zhu, Chen, & Yang, 2025). Their functional versatility—encompassing direct cytotoxicity, dendritic cell activation, and reprogramming of immunosuppressive tumor environments—sets the stage for their high therapeutic potential.
Types and Mechanisms of Action of iNKT Cells
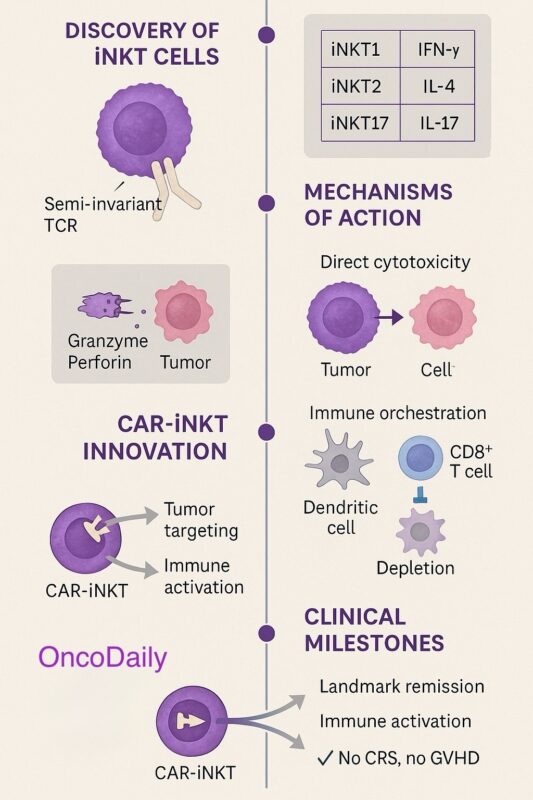
iNKT cells are functionally diverse despite sharing a semi-invariant T-cell receptor that recognizes CD1d-presented lipid antigens. In both humans and mice, they are broadly categorized into three subsets based on transcription factor expression profiles and cytokine production.
The iNKT1 subset expresses the transcription factor T-bet and predominantly produces interferon-gamma (IFN-γ). These cells exhibit a strong Th1-like phenotype and play a leading role in anti-tumor immunity due to their potent cytotoxic potential. The iNKT2 subset, characterized by expression of GATA3, secretes interleukin-4 (IL-4) and interleukin-13 (IL-13). While they contribute to immune regulation and tissue homeostasis, iNKT2 cells appear to play a less direct role in tumor control. The iNKT17 subset expresses RORγt and secretes interleukin-17 (IL-17). This subset is more closely linked to mucosal immunity and inflammation and its role in cancer is less well defined.
Among these subsets, iNKT1 cells are particularly important for cancer immunotherapy, given their Th1-skewed cytokine profile and ability to promote cytotoxic immune responses (Yin-Ruide, Zhu, Chen, & Yang, 2025).
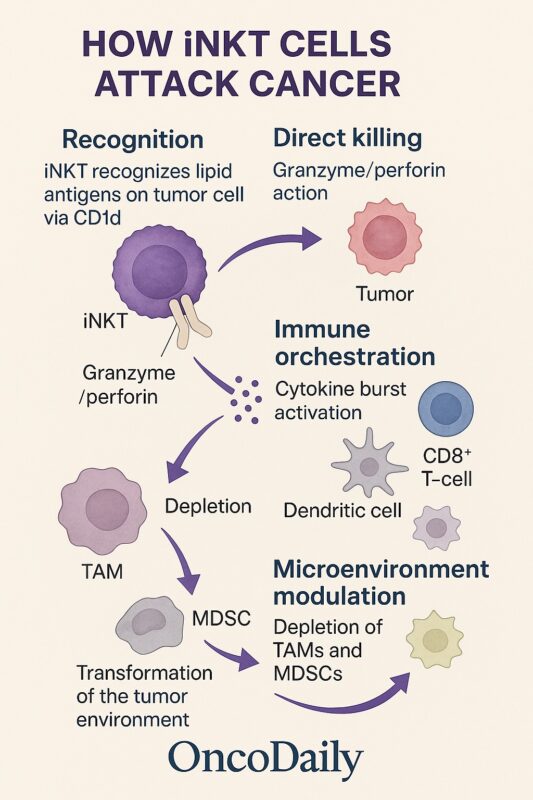
Direct Cytotoxic Mechanisms
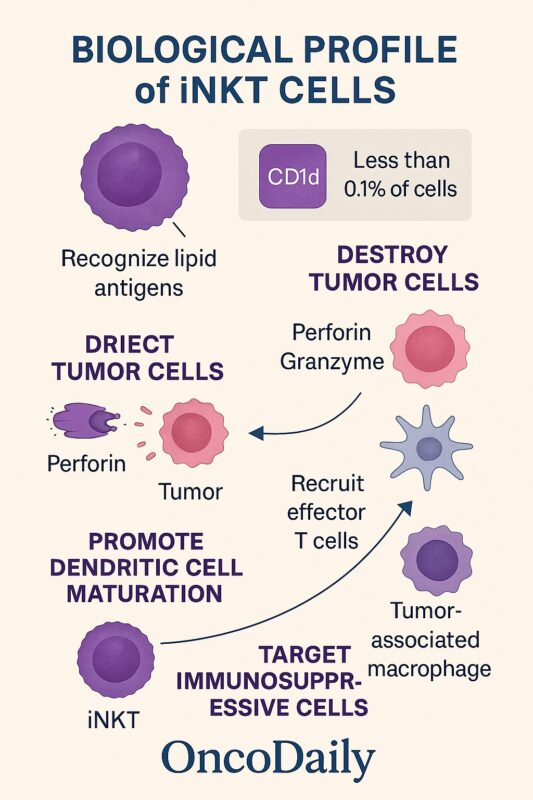
A key feature of iNKT cells is their ability to mediate direct cytotoxicity. Upon activation, iNKTs rapidly deploy granules containing perforin and granzymes, inducing apoptosis in tumor cells that express CD1d. Additionally, iNKTs can trigger Fas–Fas ligand (FasL) interactions on susceptible tumor cells, leading to caspase-dependent programmed cell death (Heczey et al., 2022). These mechanisms enable iNKTs to act as immediate effectors against malignant cells while also setting the stage for broader immune responses.
Immune-Orchestrating Functions
In addition to their direct cytotoxic activity, iNKTs function as immune system orchestrators. Their rapid secretion of cytokines such as IFN-γ and IL-4 enhances the maturation and antigen-presenting capacity of dendritic cells through CD40–CD40L interactions. Mature dendritic cells, in turn, prime tumor-specific CD8⁺ cytotoxic T lymphocytes, amplifying the overall anti-tumor immune response.
iNKTs also recruit and activate natural killer (NK) cells, expanding the network of cytotoxic effectors mobilized against tumors (Yin-Ruide et al., 2025). This broad immunostimulatory role distinguishes iNKTs from other immune effectors such as NK cells or conventional CD8⁺ T cells, which rely more narrowly on direct recognition and killing.
Modulation of the Tumor Microenvironment
A defining advantage of iNKT cells in cancer therapy is their capacity to remodel the tumor microenvironment. Many solid tumors are rich in immunosuppressive cells, particularly tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), which suppress effective anti-tumor immunity. iNKTs can target and deplete these populations, reducing local immune suppression and creating a more permissive environment for immune cell infiltration and function.
By altering the cytokine balance within the tumor milieu and eliminating immunosuppressive stromal components, iNKTs help restore immune surveillance and improve the efficacy of both innate and adaptive anti-cancer responses (Traversing bench to bedside: Clinical translation of invariant NKT cell therapies, 2024).
Biological Profile of iNKT Cells
iNKTs account for less than 0.1% of circulating T cells but have outsized immunoregulatory roles. Their CD1d restriction allows MHC-independent antigen recognition, providing a broad surveillance mechanism against malignancy and infection (Yin-Ruide et al., 2025). Upon activation, iNKTs kill tumor cells through perforin/granzyme pathways, promote dendritic cell maturation, and recruit effector T cells into tumors. Importantly, iNKTs also target immunosuppressive tumor-associated macrophages and myeloid-derived suppressor cells, making them particularly attractive for solid tumors with a hostile microenvironment.
Preclinical Evidence of Anti-Tumor Activity
Preclinical models have provided strong proof-of-concept for iNKT cell therapy. In murine solid tumor studies, adoptive transfer of ex vivo expanded iNKTs or administration of α-GalCer-loaded dendritic cells significantly reduced tumor burden and enhanced anti-tumor T-cell infiltration (Yin-Ruide et al., 2025). Moreover, iNKT cells have shown the ability to prevent graft-versus-host disease (GVHD) in allogeneic transplantation models while maintaining graft-versus-leukemia activity (Traversing bench to bedside: Clinical translation of invariant NKT cell therapies, 2024). These studies demonstrate both the safety and versatility of iNKT cells as immune effectors.
CAR-iNKT Therapy: A Technological Leap
Genetically engineering iNKT cells with chimeric antigen receptors (CARs) enhances their tumor specificity while retaining their innate immunomodulatory advantages. Preclinical studies of CAR-iNKTs targeting GD2 in neuroblastoma models reveal superior tumor clearance compared to CAR-T cells. Not only do CAR-iNKTs eradicate tumor cells, but they also reduce tumor-associated macrophages, which contribute to immune suppression in the tumor microenvironment (Heczey et al., 2022). Additional CAR targets include CD19 for B-cell malignancies and BCMA for multiple myeloma, reflecting broad therapeutic potential.
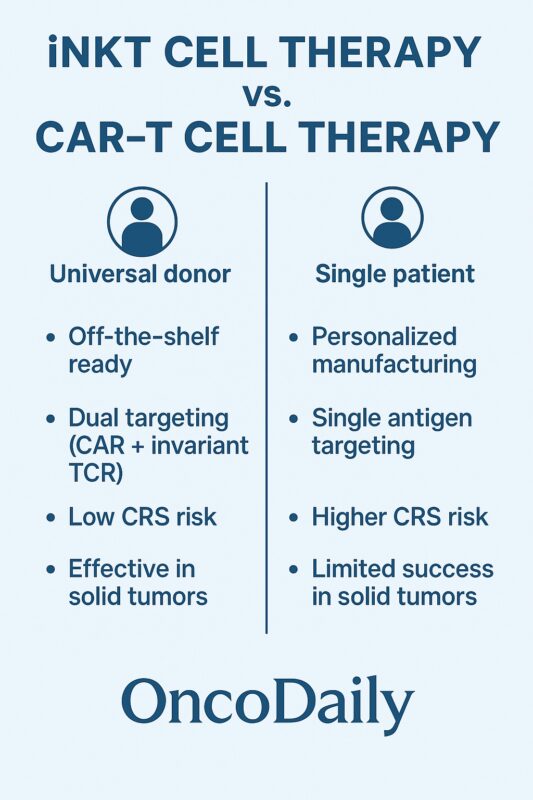
Why CAR-iNKTs May Outperform CAR-T Cells
Several features make CAR-iNKTs unique compared to CAR-T cells. Their CD1d restriction minimizes the risk of GVHD, enabling true off-the-shelf therapies without the need for complex TCR or HLA gene editing (Yin-Ruide et al., 2025). CAR-iNKTs also exhibit dual killing mechanisms: through their CAR for antigen-specific targeting and through their invariant TCR for recognition of CD1d-expressing cells. This duality may drive broader tumor control and immune priming. Importantly, CAR-iNKTs show reduced toxicity risks, including lower rates of cytokine release syndrome and neurotoxicity, based on preclinical and early clinical trial data (Heczey et al., 2022).
Early Clinical Trial Insights
Human trials of iNKT cell therapies began with α-GalCer-pulsed dendritic cell vaccines to activate endogenous iNKTs in advanced cancers like melanoma and non-small cell lung cancer. These phase I studies demonstrated feasibility and immune activation but lacked substantial clinical responses, partly due to low baseline iNKT numbers (Traversing bench to bedside: Clinical translation of invariant NKT cell therapies, 2024).
The advent of CAR-iNKT therapy represents a major advance. A phase I trial of GD2-CAR-IL15 iNKTs in children with neuroblastoma showed a favorable safety profile, with tumor infiltration and an objective response rate around 25% and no severe cytokine release syndrome or GVHD (Traversing bench to bedside: Clinical translation of invariant NKT cell therapies, 2024). Ongoing trials are exploring CAR-iNKTs for B-cell malignancies and solid tumors, reflecting accelerating clinical momentum.
Breakthrough Cases
MiNK Therapeutics recently reported a groundbreaking case of complete, sustained remission (>2 years) in a patient with metastatic, treatment-refractory testicular cancer following a single allogeneic iNKT therapy, agenT-797, administered alongside nivolumab (MiNK Therapeutics Announces Complete Remission, 2025). The patient had previously failed platinum-based chemotherapy, autologous stem cell transplantation, and checkpoint inhibitors. Notably, there were no reports of cytokine release syndrome or GVHD, and donor-derived iNKTs persisted for up to six months post-infusion. Complementary early data from a Phase II gastric cancer cohort showed treatment-induced tumor shrinkage and immune activation, with progression-free survivals extending beyond 12 months (MiNK Therapeutics Announces Complete Remission, 2025). This case constitutes the first peer-reviewed account of complete remission in a metastatic solid tumor treated solely with allogeneic iNKTs, representing a meaningful inflection point in cellular immunotherapy.
Additionally, MiNK shared early Phase 2 gastric cancer data showing promising immune activation and tumor infiltration, with several patients achieving survival beyond 12 months. In one case, a metastatic gastric tumor shrank by 42%, resulting in nine months of progression-free survival. These outcomes bolster evidence that off-the-shelf allo‑iNKT therapies can deliver meaningful, safe responses in both solid and hematologic tumors.

Read About Breakthrough iNKT Cell Therapy Achieves Complete Remission in Metastatic Germ Cell Tumor on OncoDaily
Challenges in Manufacturing and Optimization
Despite their promise, iNKT therapies face hurdles. The rarity of iNKTs requires effective ex vivo expansion protocols using cytokines like IL-2 and IL-15 to generate sufficient therapeutic doses (Heczey et al., 2022). Subset optimization is another frontier: CD4⁻ iNKTs are believed to have greater cytotoxicity and Th1 bias, potentially enhancing tumor killing (Traversing bench to bedside: Clinical translation of invariant NKT cell therapies, 2024). Host-versus-graft rejection remains a concern for allogeneic products, necessitating exploration of gene editing to enhance immune evasion and persistence.
Next-Generation Innovations
New strategies promise to improve iNKT therapy durability and potency. Induced pluripotent stem cell (iPSC)–derived iNKTs offer a scalable, uniform source for manufacturing. Smart CAR designs incorporating inducible activation systems, cytokine co-expression (e.g., IL-15), and safety switches are under investigation to enhance persistence while minimizing toxicity (Yin-Ruide et al., 2025). Combinatorial approaches—such as pairing CAR-iNKTs with immune checkpoint inhibitors or therapeutic vaccines—may further potentiate anti-tumor efficacy, particularly in solid tumors where single-modality therapies often fail.
Future Directions
The field of iNKT cell therapy is advancing rapidly, with several ongoing trials and preclinical programs exploring new targets and innovative manufacturing approaches. Key areas of focus include improving cell persistence in vivo, overcoming host rejection, refining subset selection, and expanding therapeutic indications beyond hematologic malignancies into solid tumors. As safety and efficacy data accumulate, iNKT-based immunotherapy may soon join the clinical mainstream as a versatile, scalable, and well-tolerated cancer treatment platform.
Invariant NKT cell therapy exemplifies the next frontier in cellular immunotherapy. With their hybrid innate-adaptive profile, ability to reshape the tumor microenvironment, and reduced toxicity profile, iNKTs address many of the limitations seen with conventional CAR-T therapies. CAR-iNKT therapies in particular offer the promise of off-the-shelf availability, dual mechanisms of tumor targeting, and lower risks of severe adverse events. Emerging clinical evidence and ongoing innovation in manufacturing, gene engineering, and combination strategies suggest that iNKT therapies will play a transformative role in cancer care over the next decade.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD